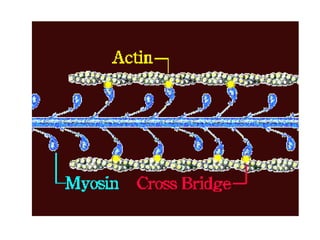
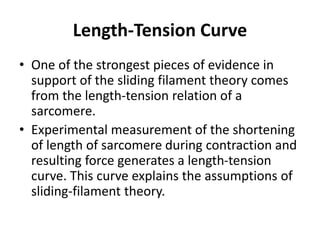
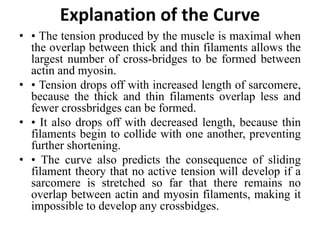
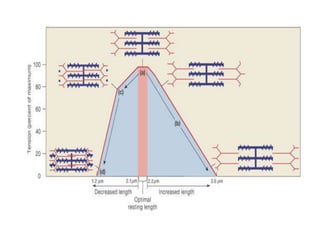
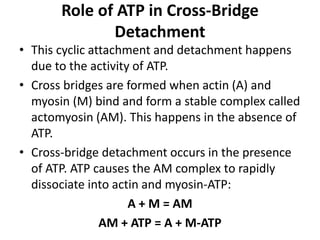
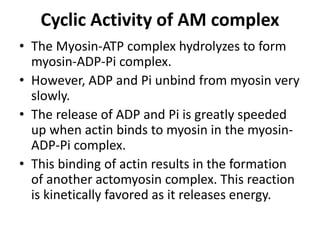
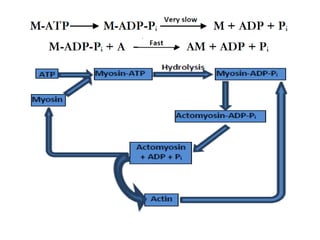
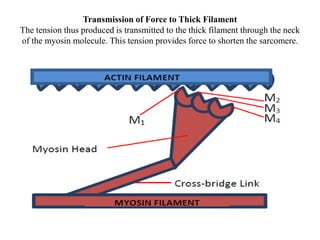
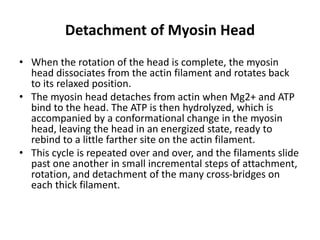
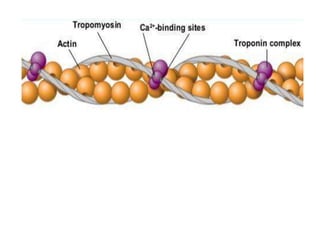
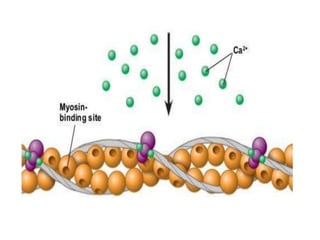
The sliding filament theory of muscle contraction proposes that during muscle contraction, actin and myosin filaments slide past each other. This occurs through a cyclic process where myosin cross-bridges attach to and detach from actin binding sites, pulling the filaments together. Calcium ions trigger muscle contraction by binding to troponin and tropomyosin, exposing actin binding sites for cross-bridge formation. An action potential leads to calcium release from the sarcoplasmic reticulum through excitation-contraction coupling, initiating the contraction process.