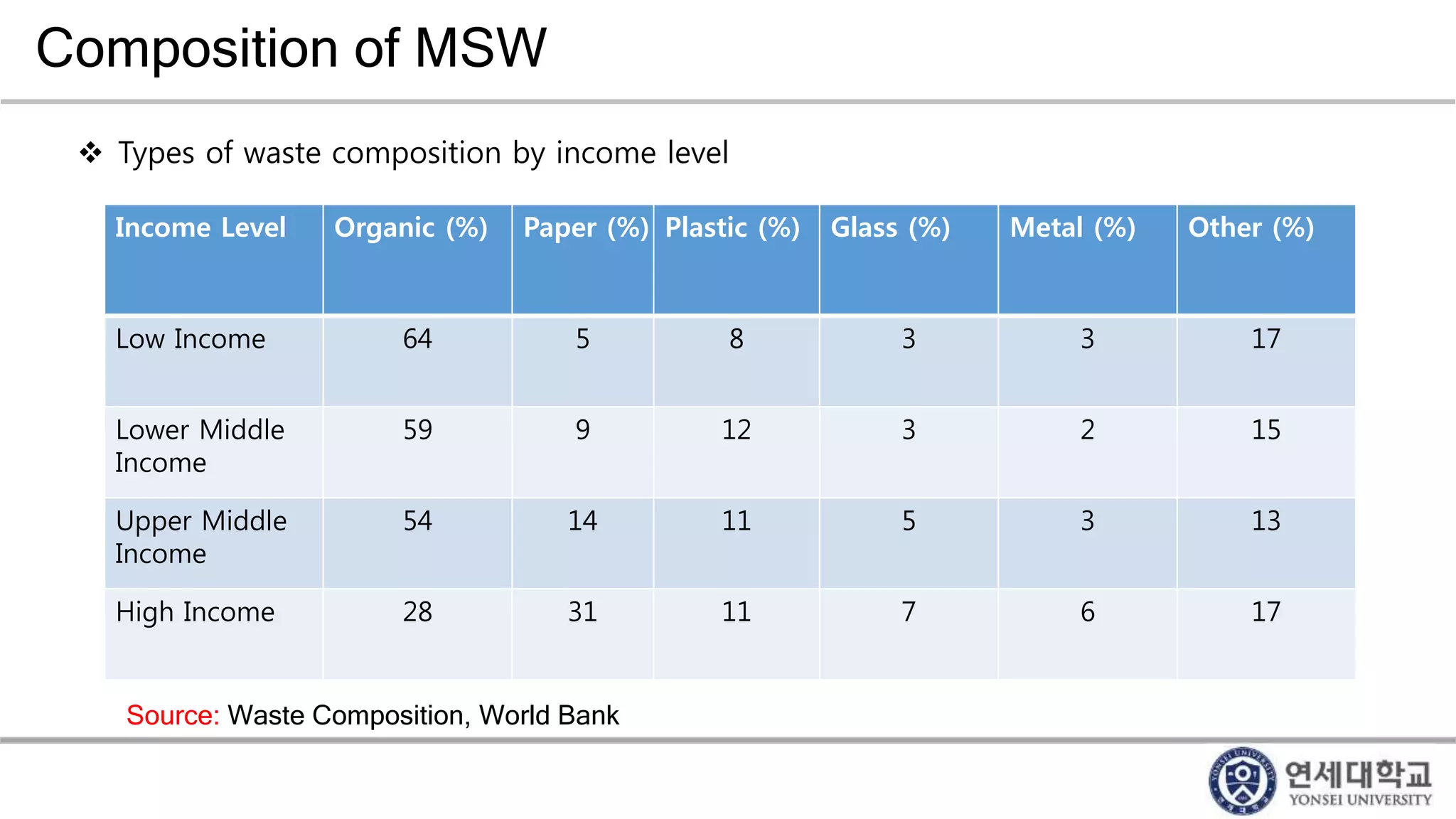
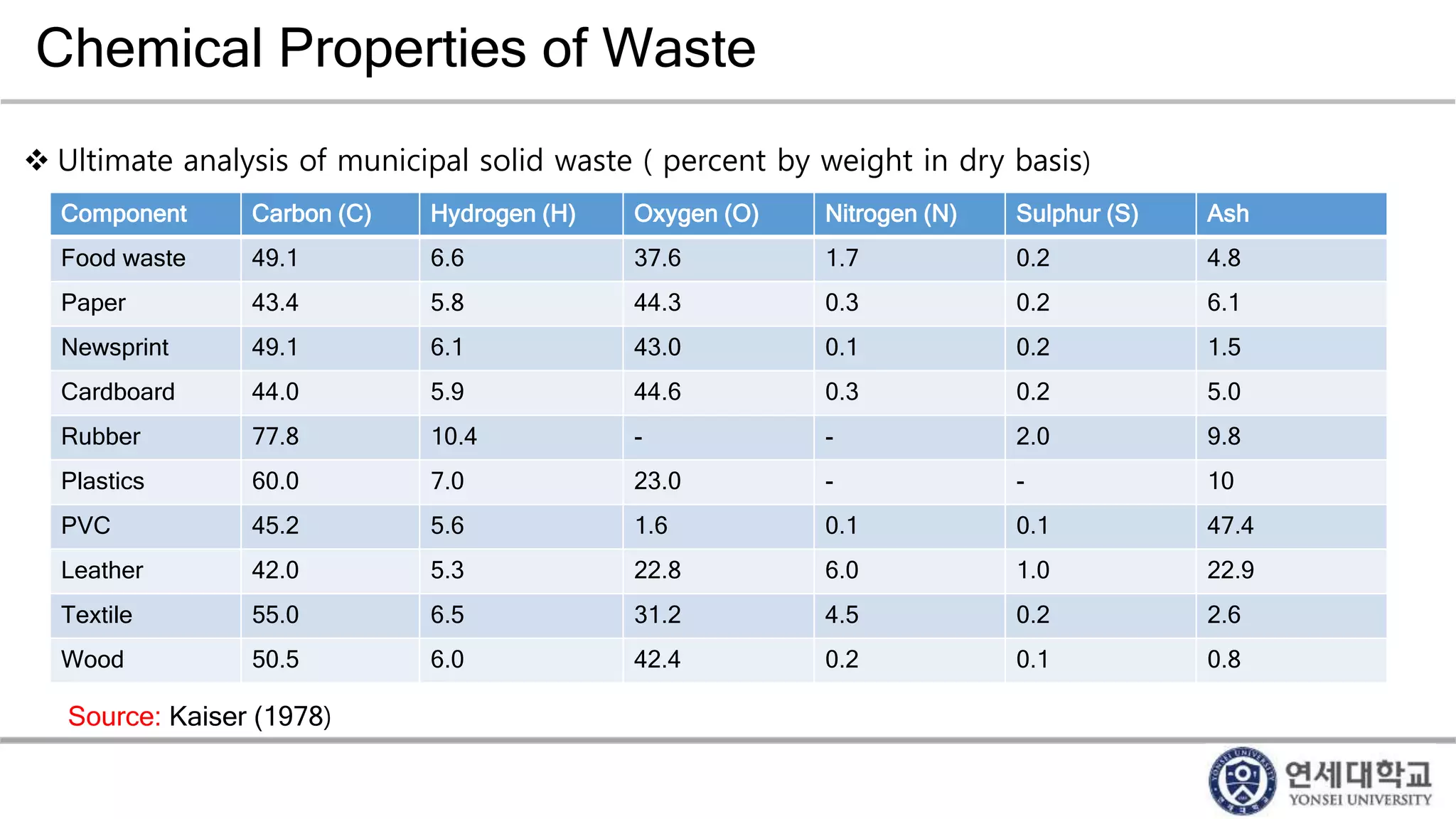
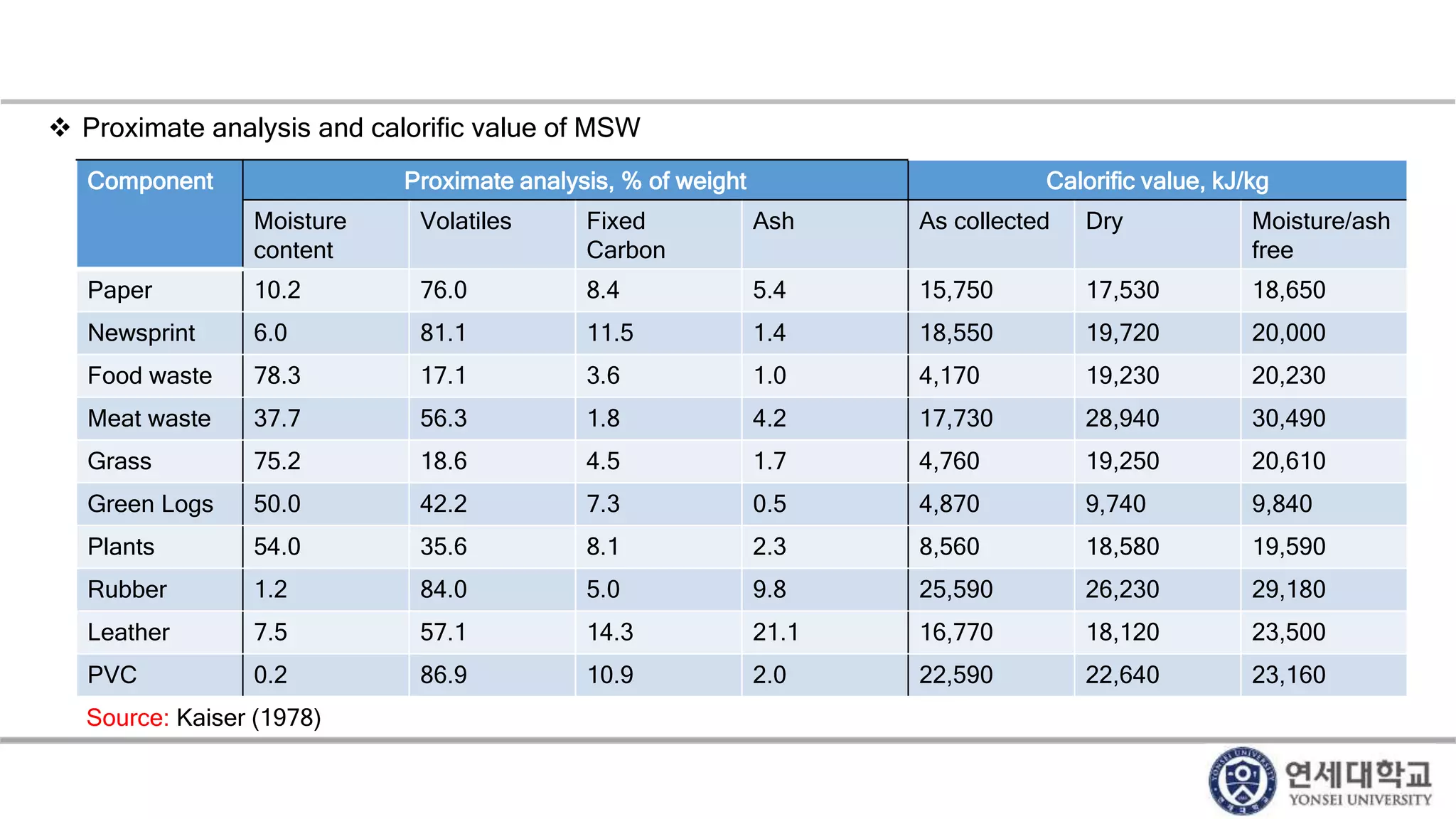
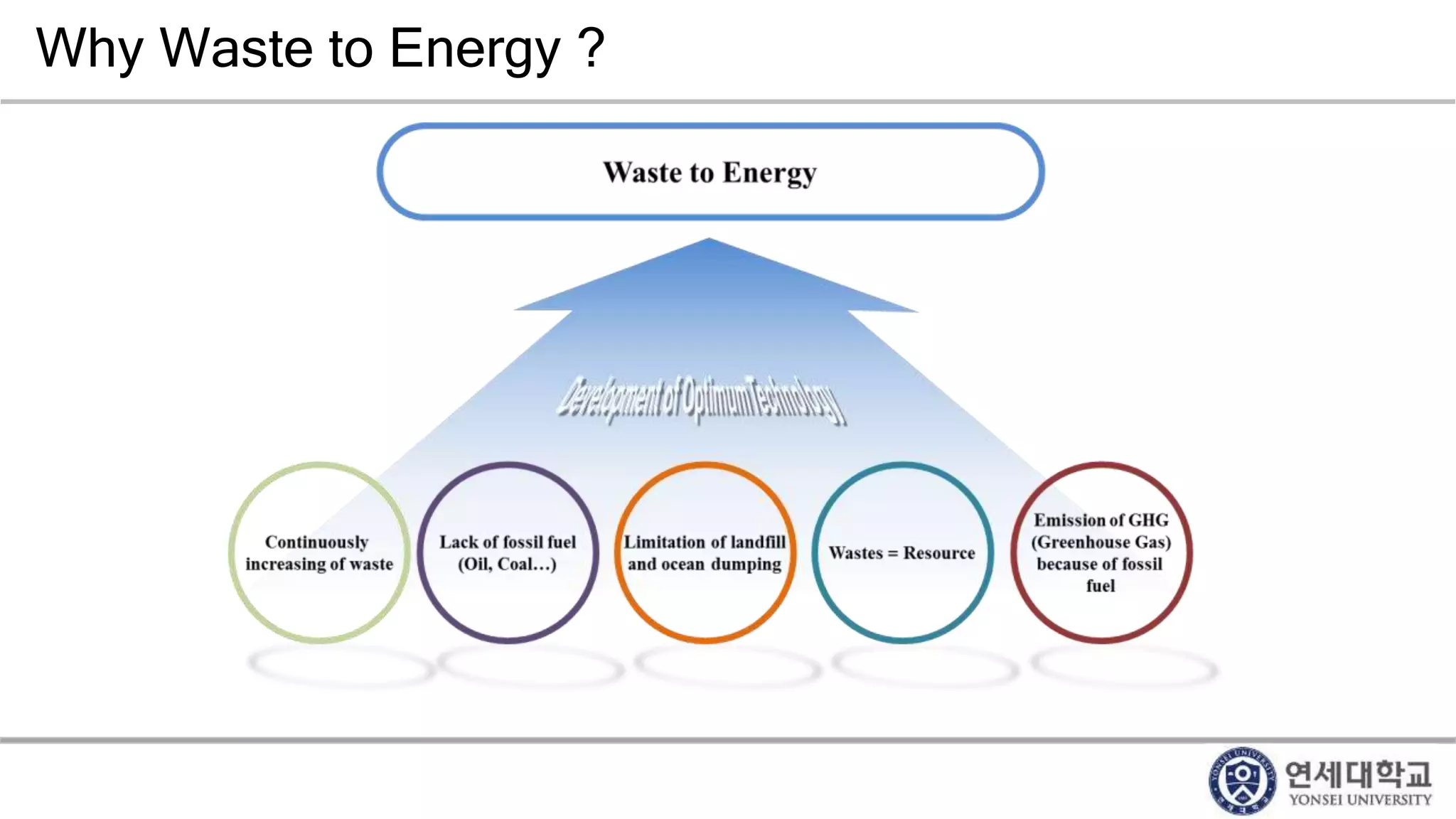
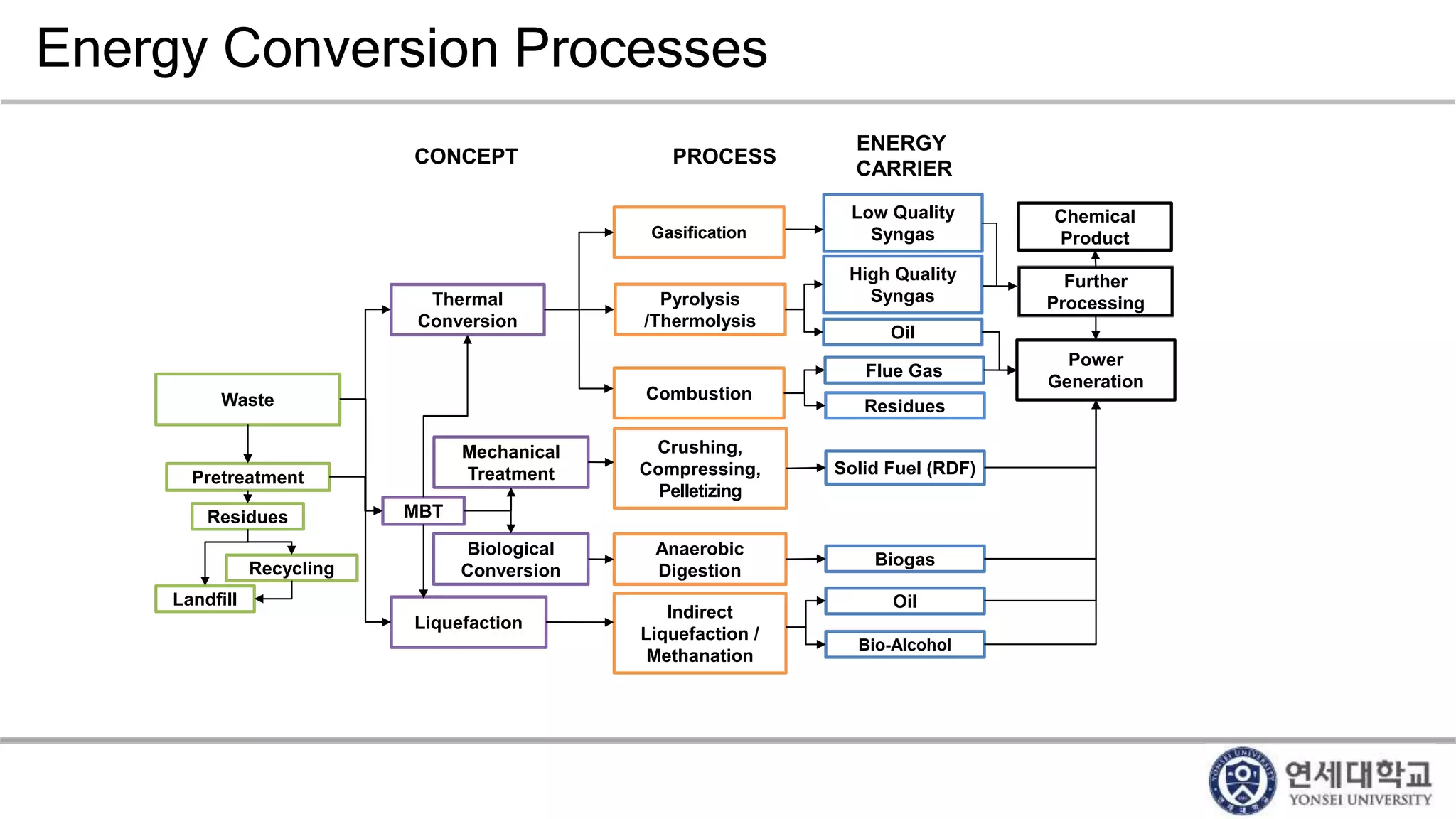
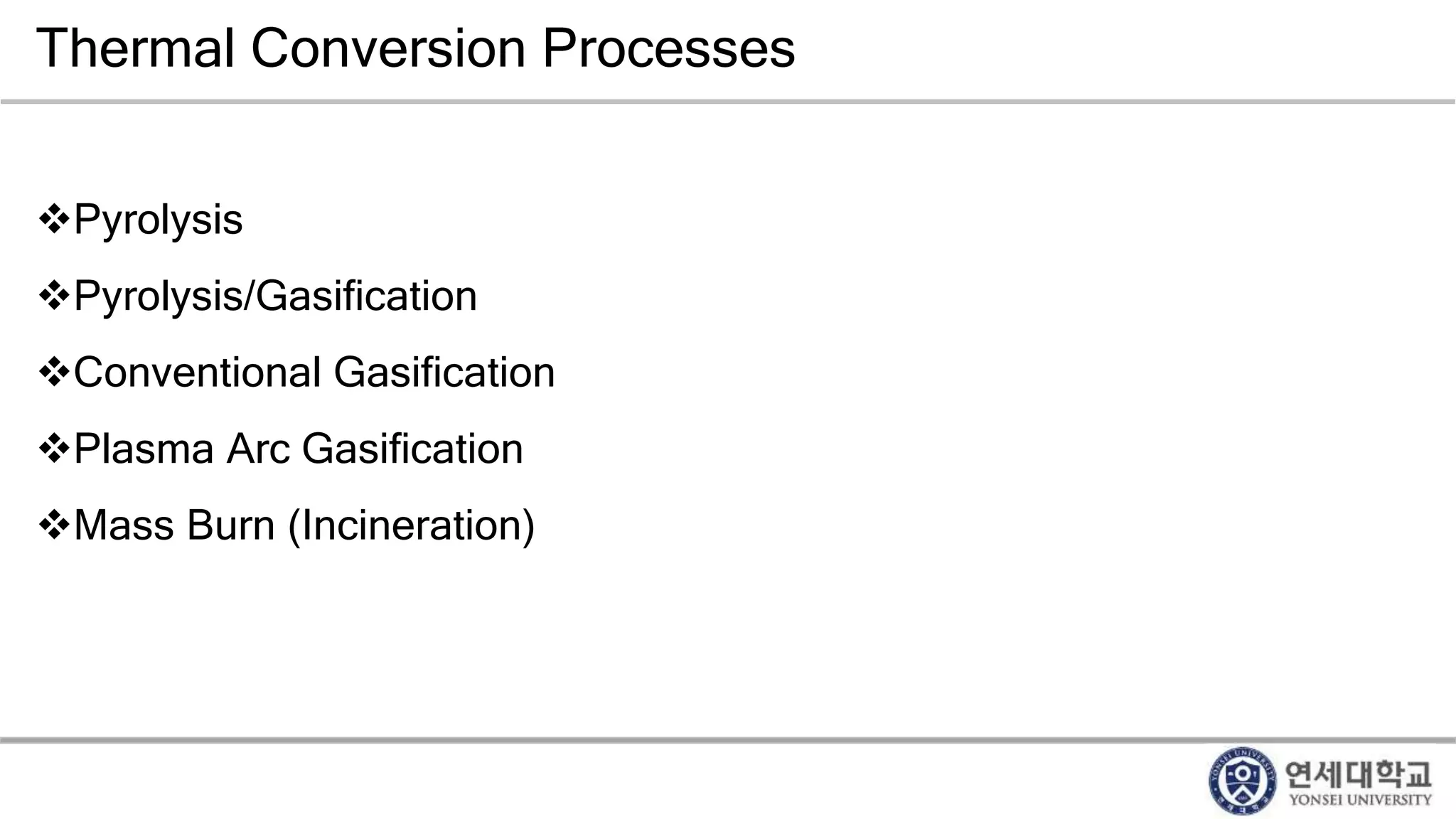
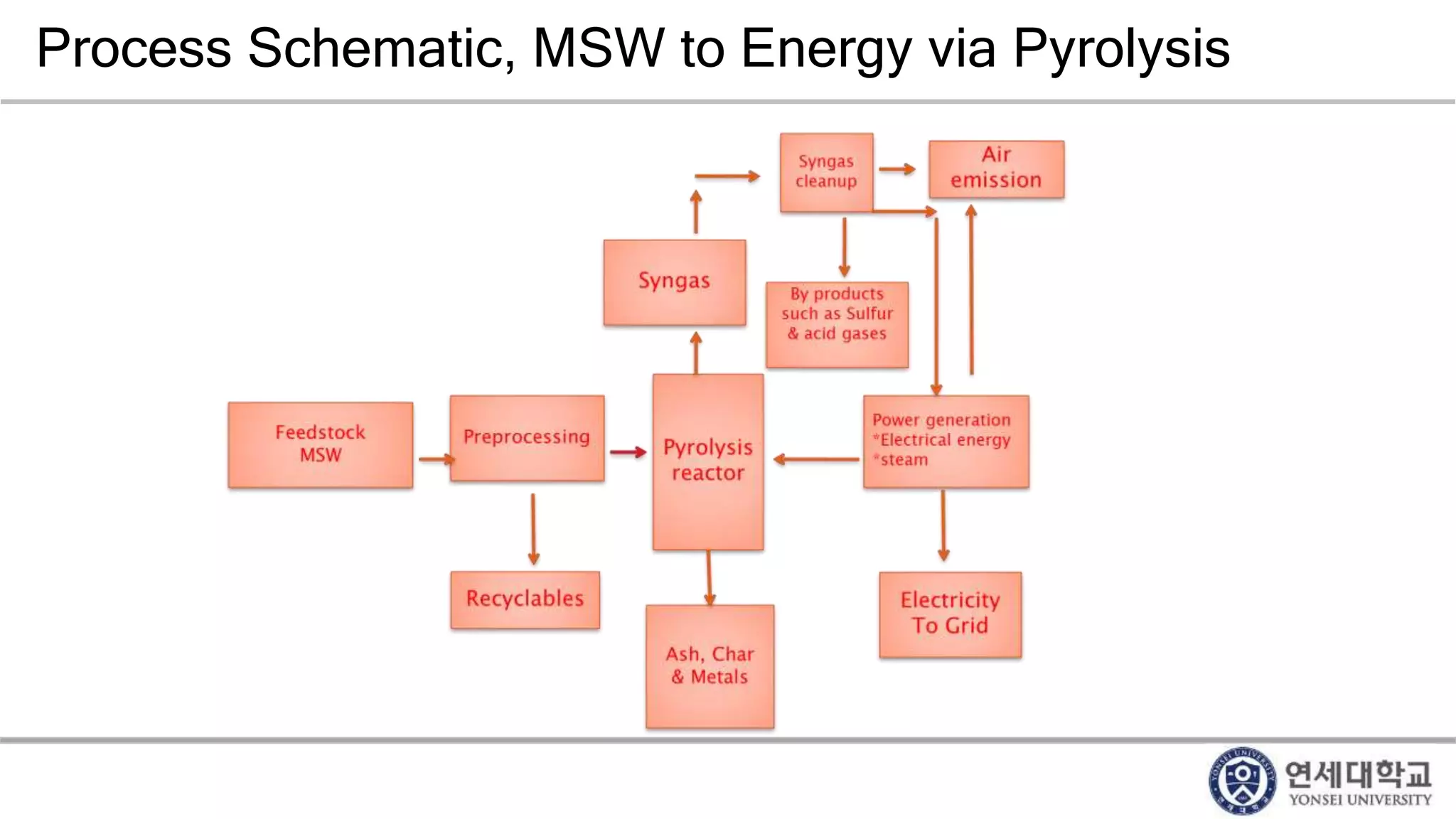
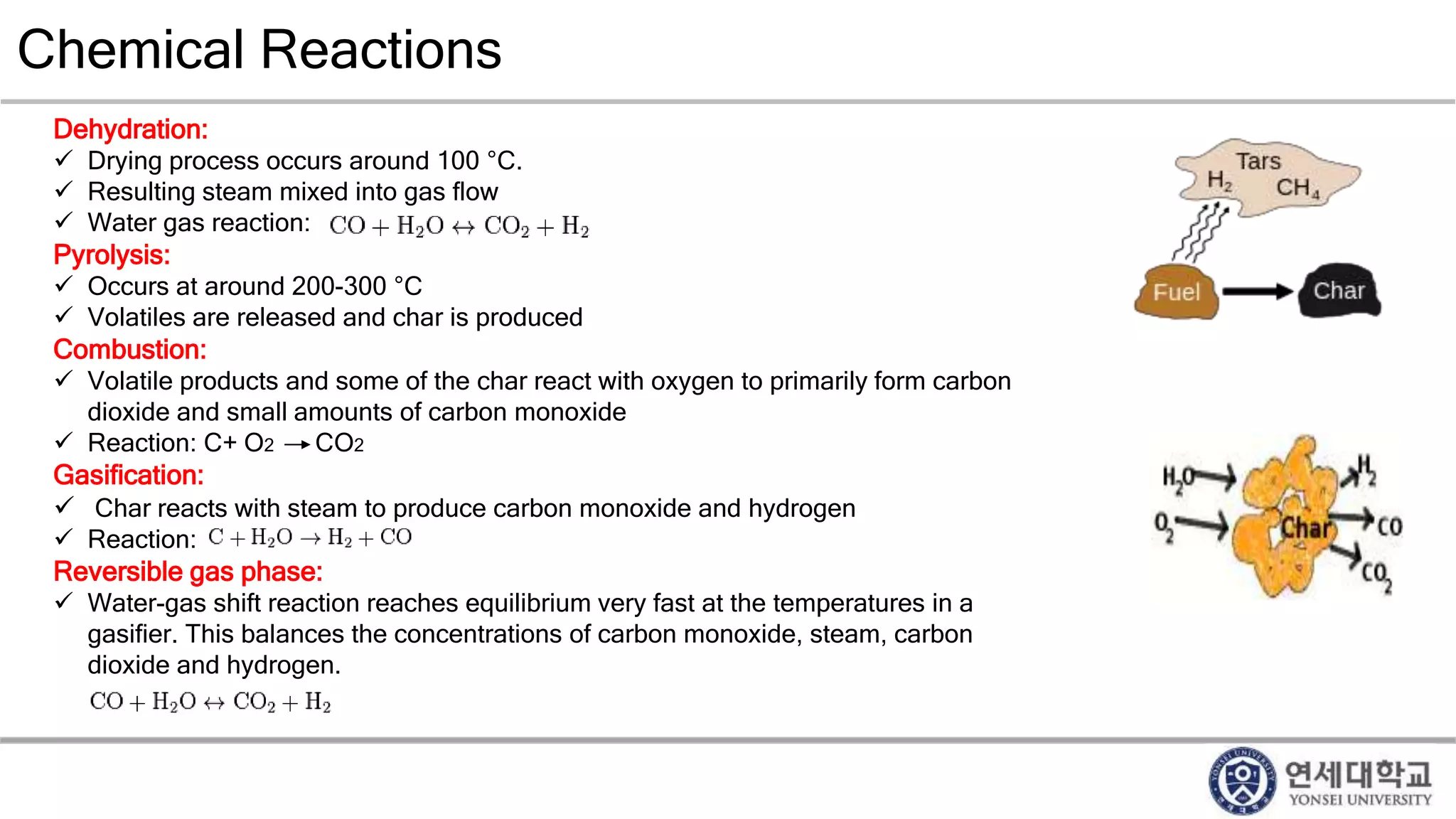
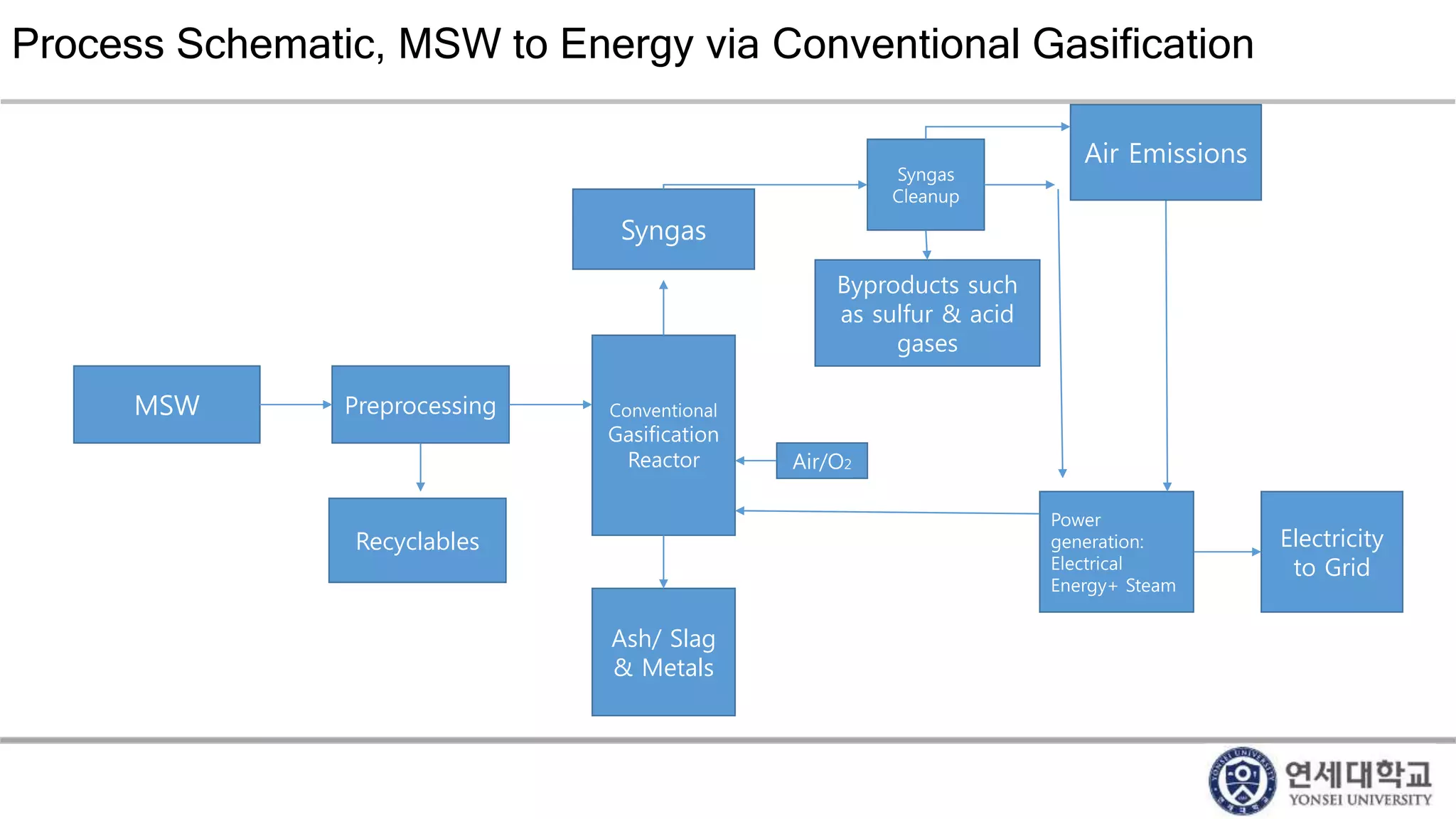
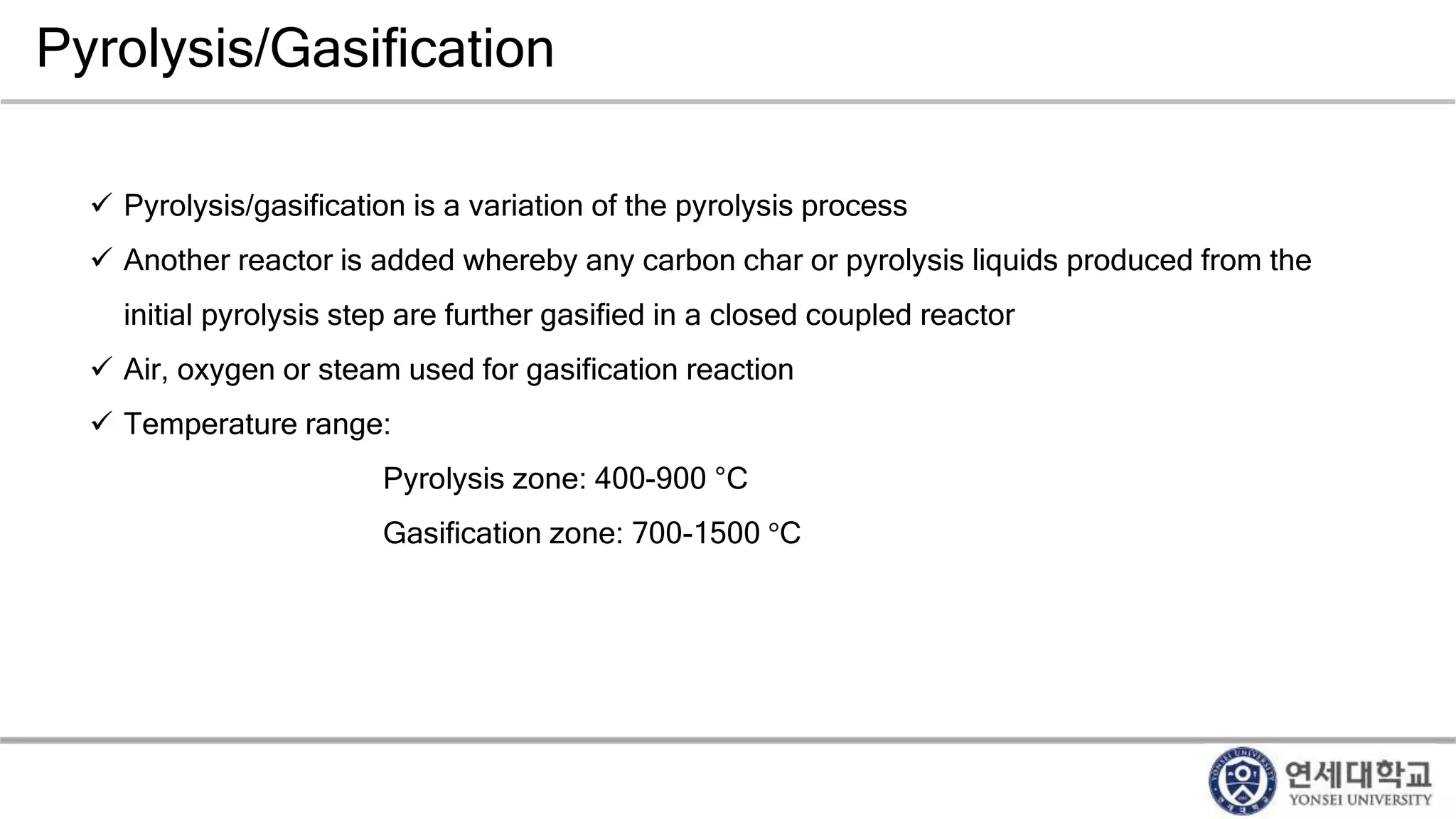
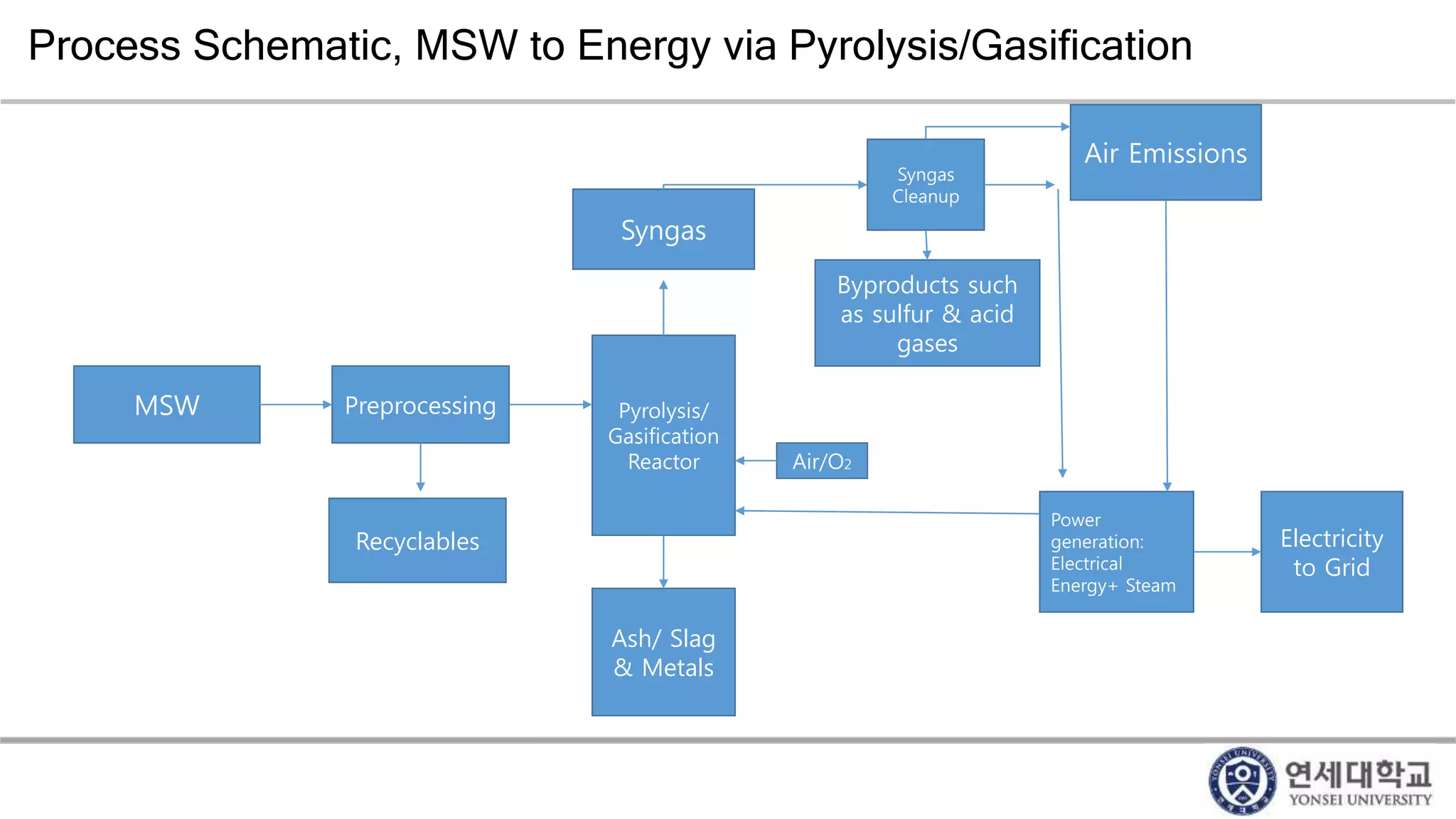
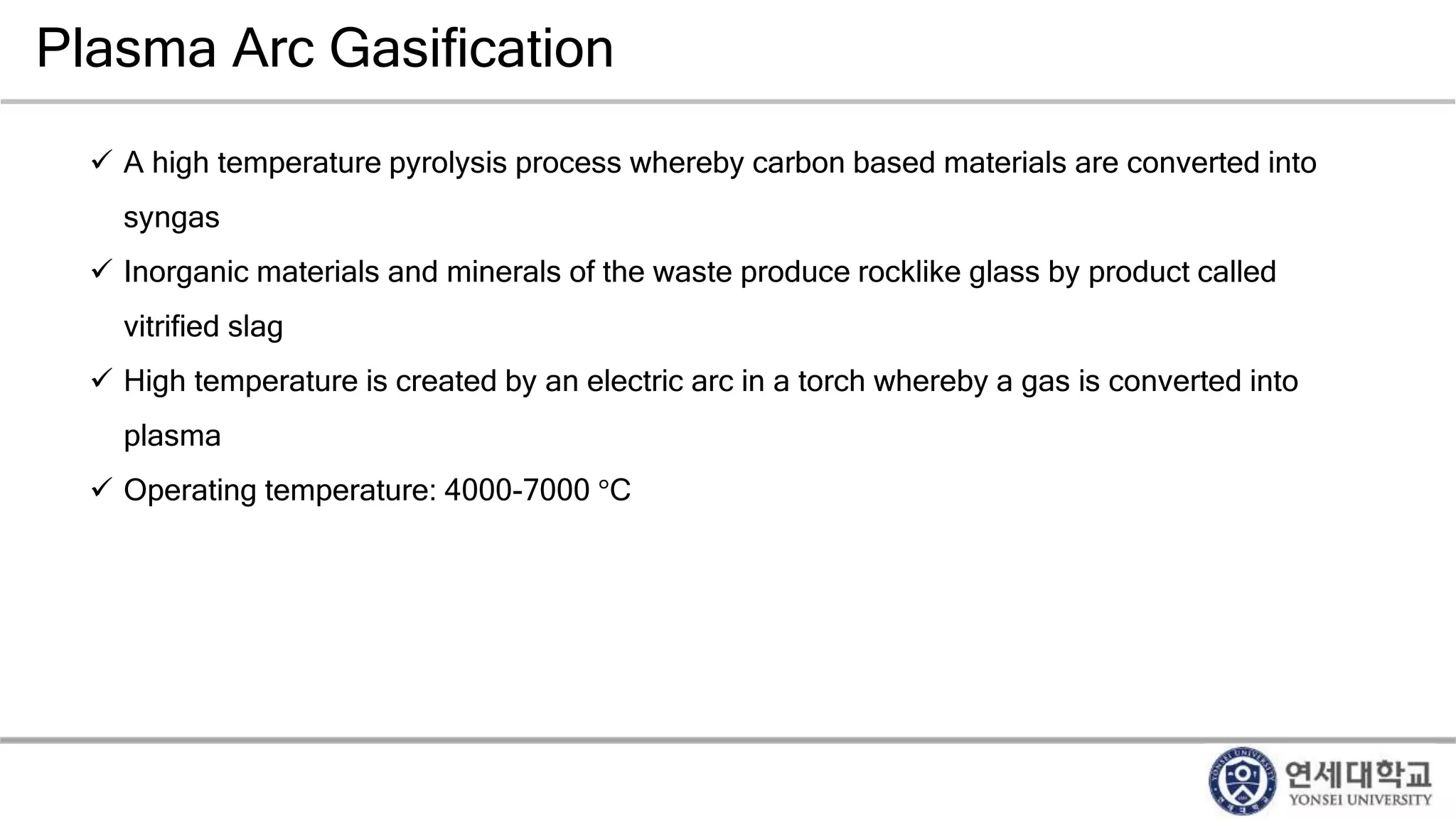
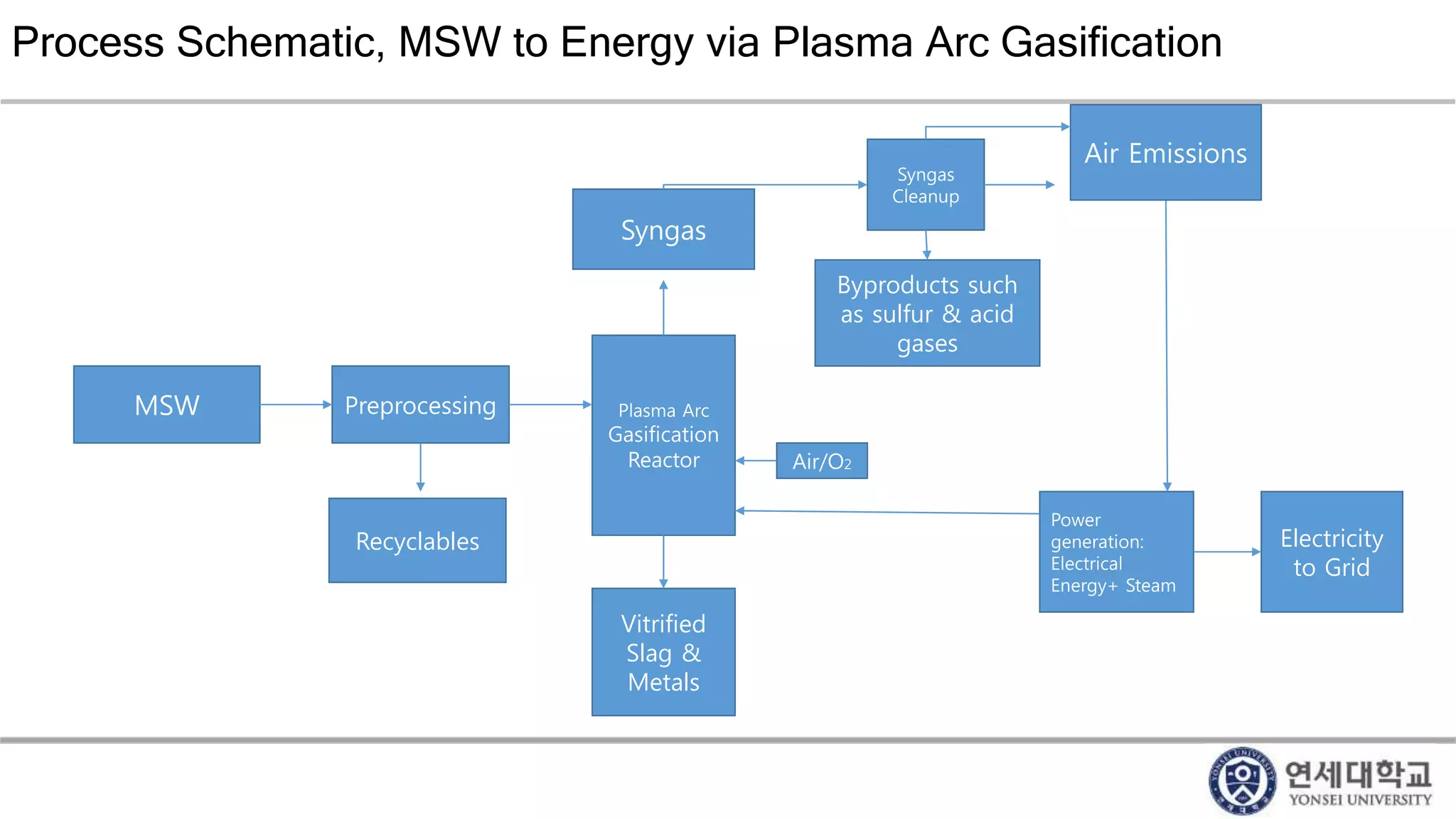
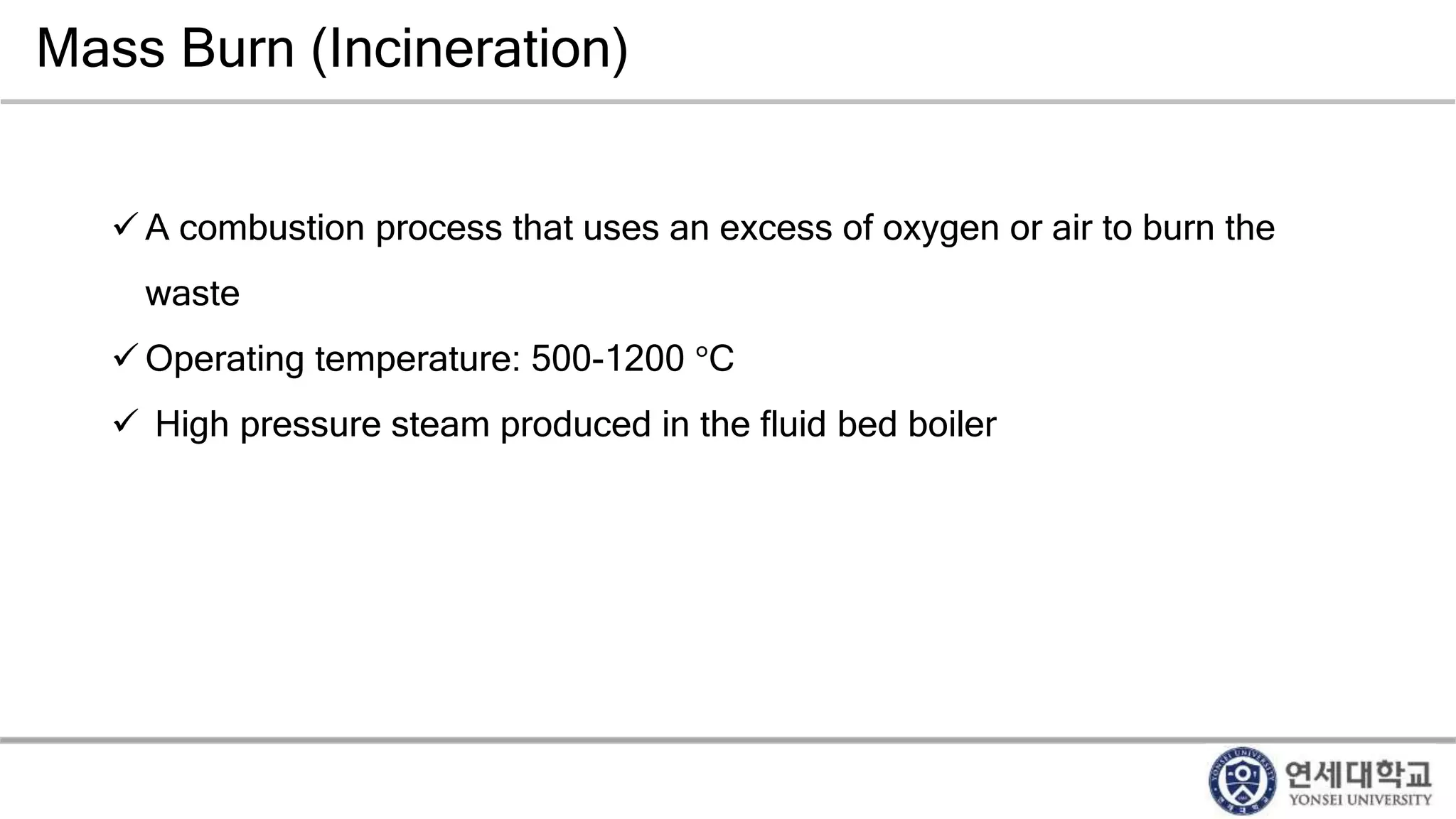
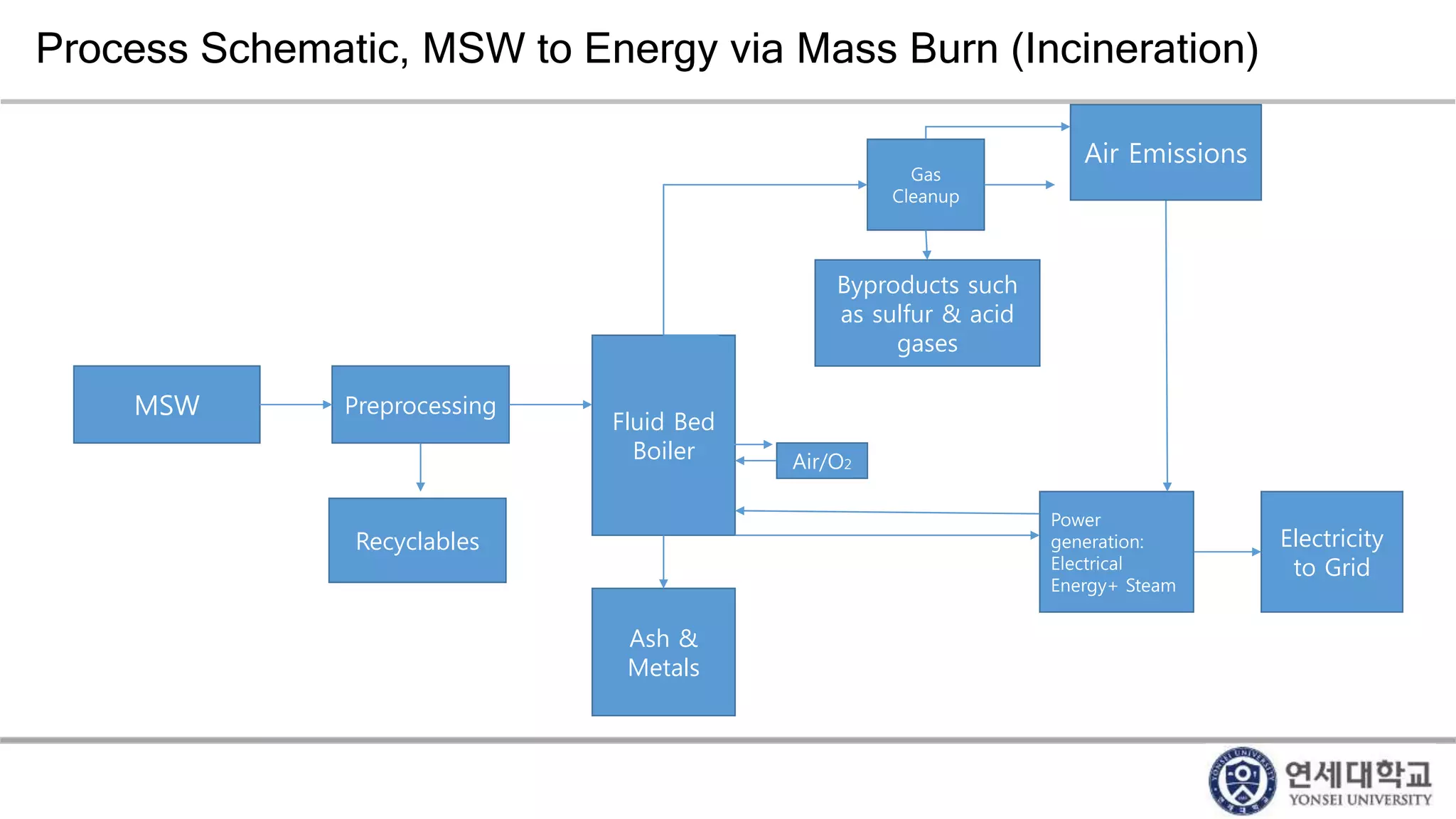
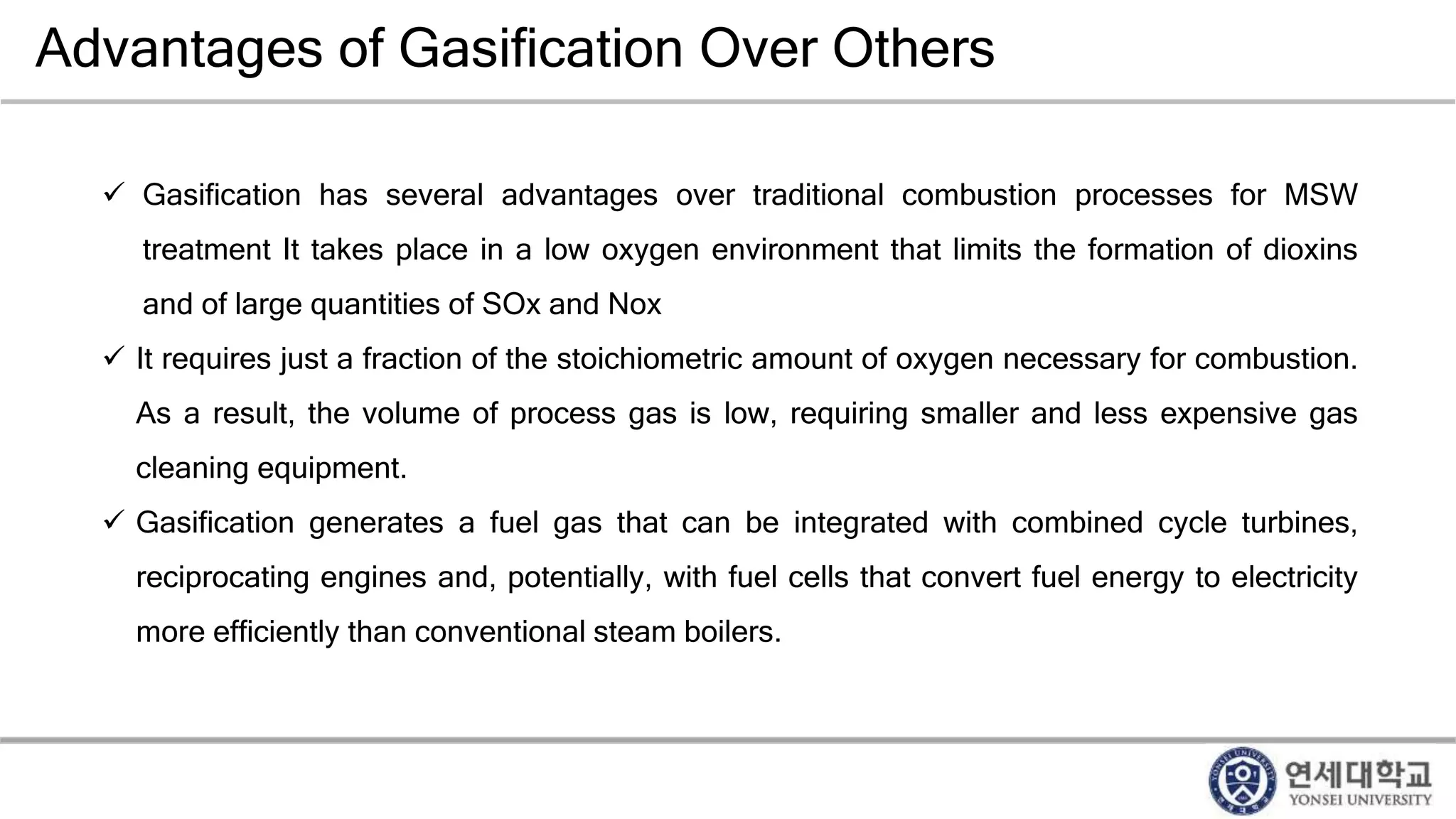
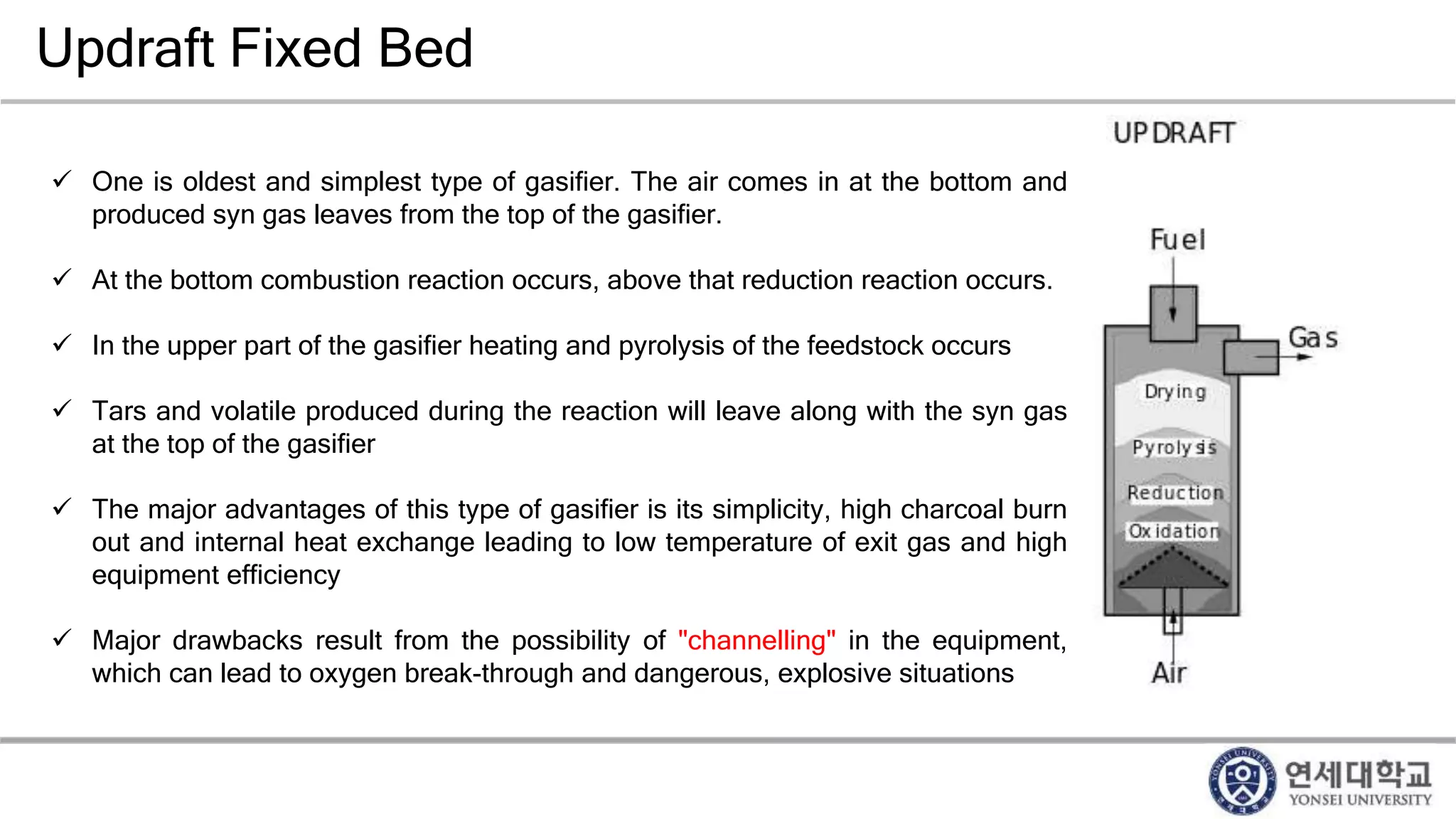
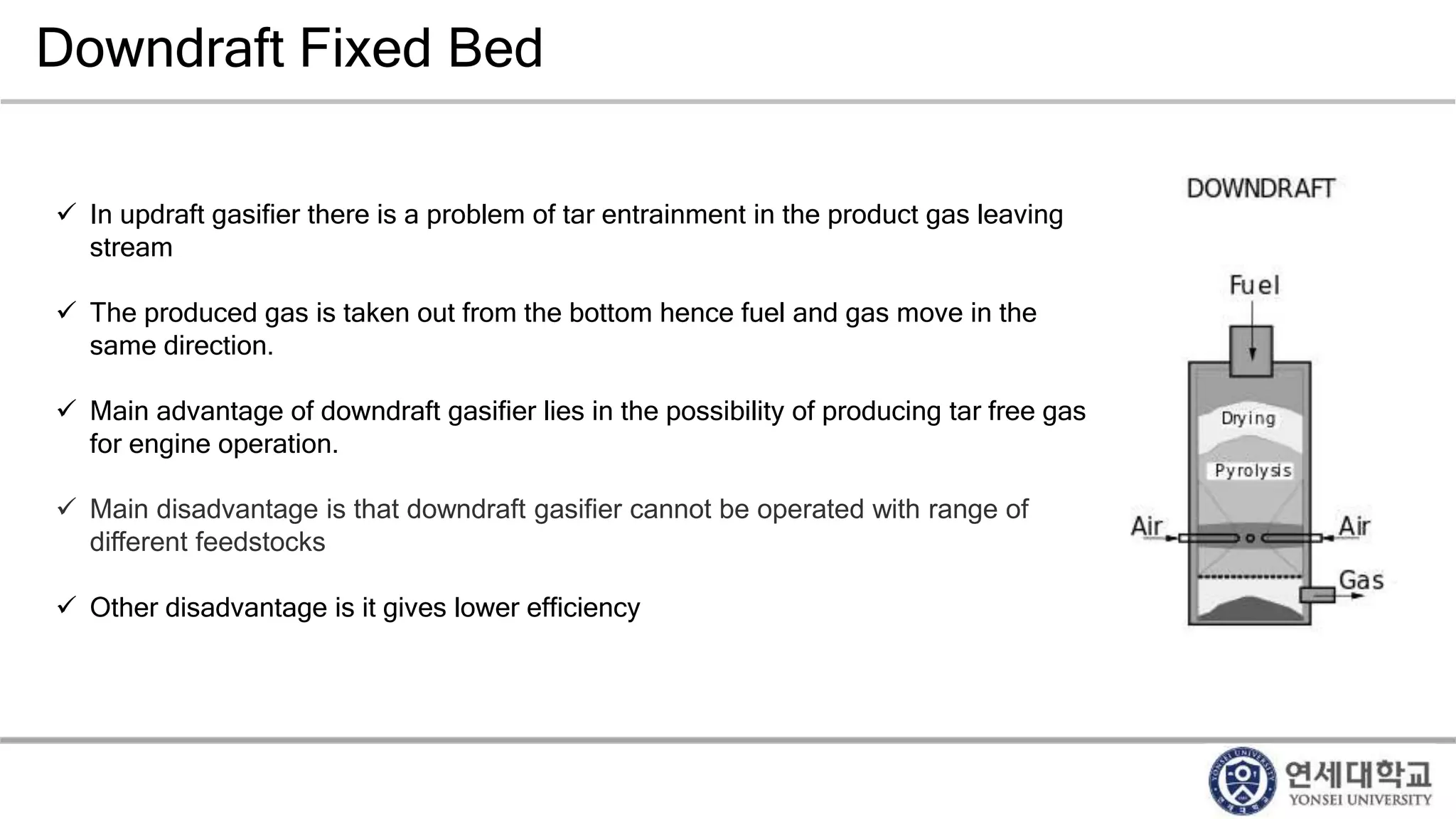
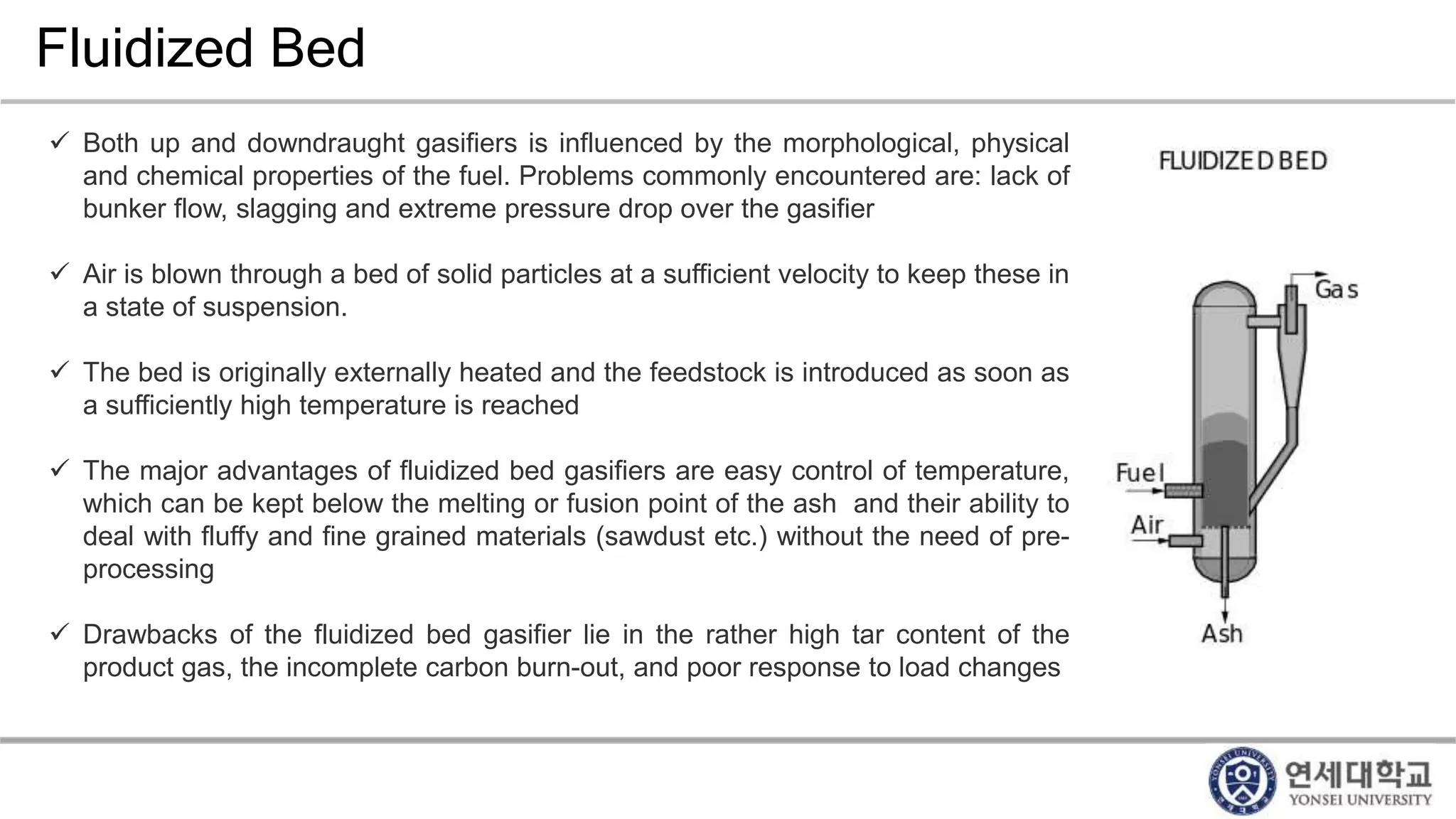
Municipal solid waste can be converted into energy through various thermal conversion processes. These include pyrolysis, gasification, plasma arc gasification, and mass burn incineration. Pyrolysis involves heating waste in an oxygen-free environment to produce syngas. Gasification also produces syngas by partially combusting waste with a controlled amount of air or oxygen. Plasma arc gasification uses an electric arc to heat waste to very high temperatures. Mass burn incineration fully combusts waste at high temperatures to produce steam. The syngas produced can be cleaned and used to generate electricity while reducing waste volumes and producing an alternative energy source from municipal solid waste.