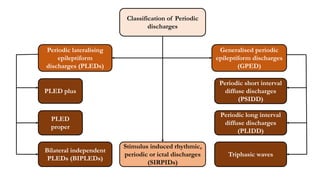
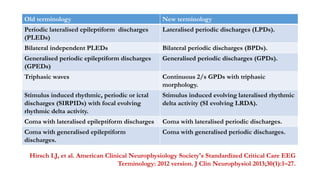
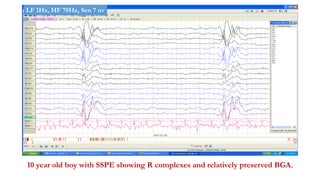
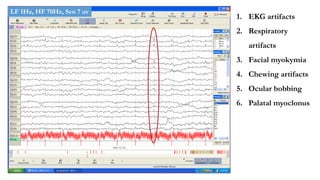
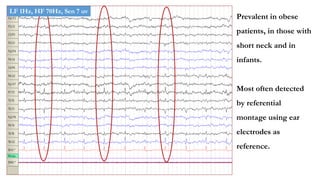
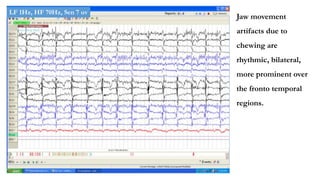
The document discusses generalized periodic discharges in EEG, detailing various types such as periodic short interval diffuse discharges (PSIDD), periodic long interval diffuse discharges (PLIDD), and triphasic waves, including their characteristics and clinical significance. It highlights the role of these discharges in indicating significant brain impairment and the potential for seizures, particularly in critically ill patients. Additionally, it covers classification, mechanisms, and associations of periodic discharges, along with specific EEG features relevant to conditions like Creutzfeldt-Jakob disease.