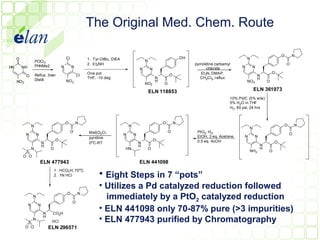
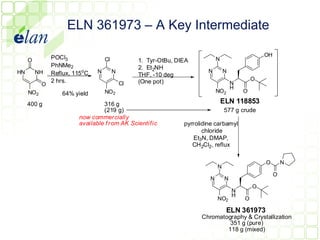
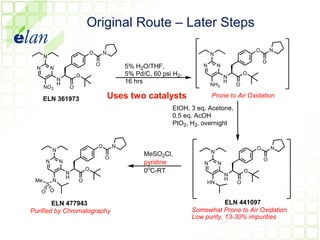
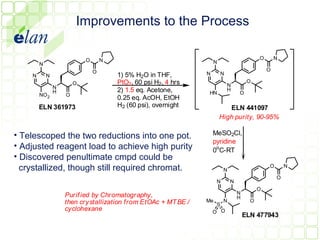
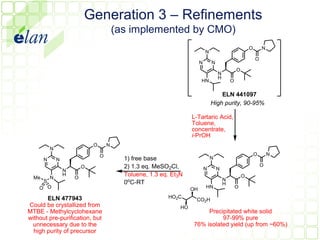
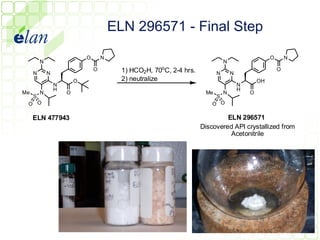
The document describes improvements made to the process for manufacturing the potential therapeutic compound ELN 296571. It summarizes the original multi-step synthesis route and key intermediate compound ELN 361973. The route was optimized to require fewer steps and produce ELN 361973 in higher yields and purity. Later, production of the important intermediate ELN 361973 was outsourced to improve the overall process.