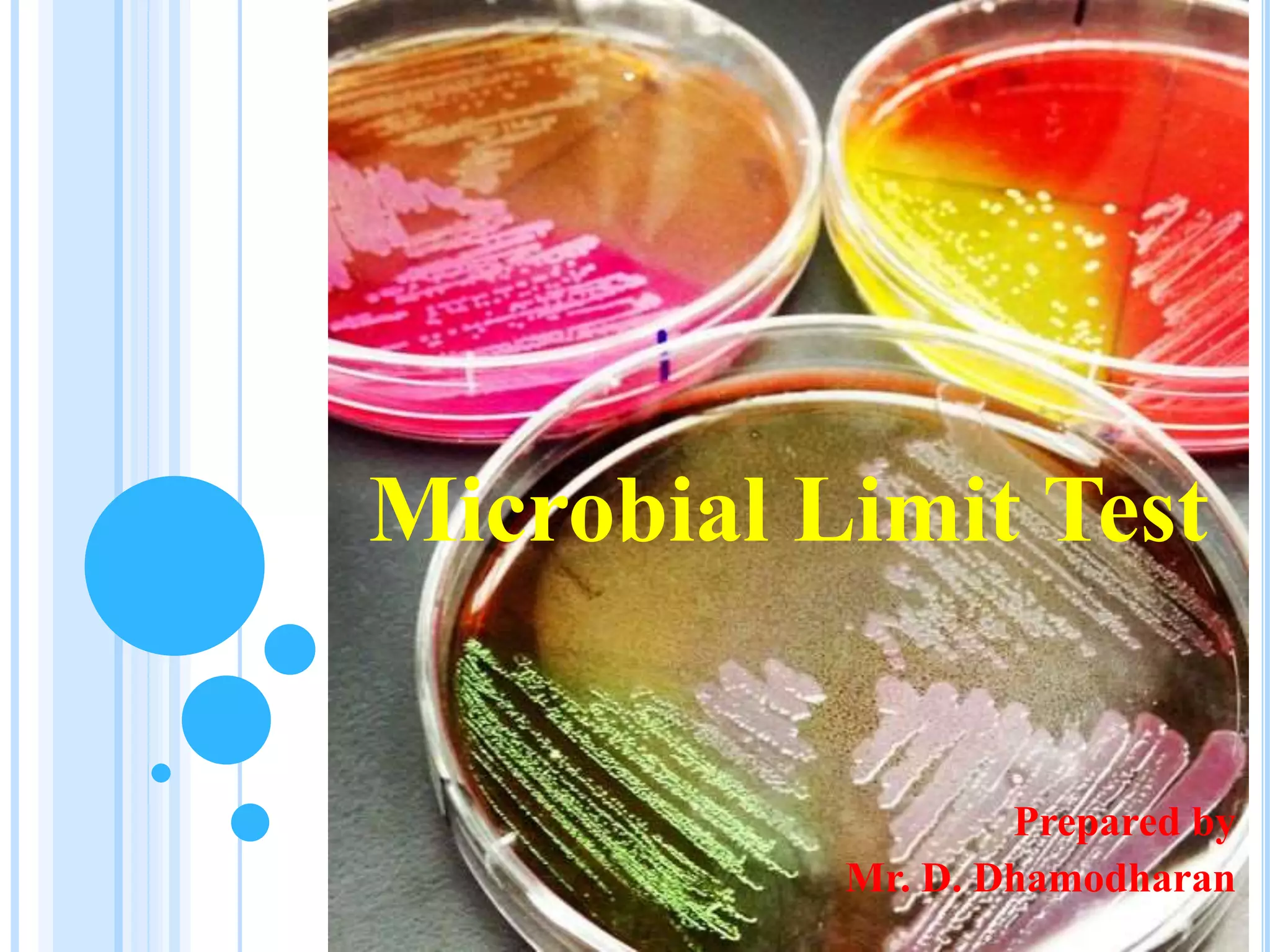
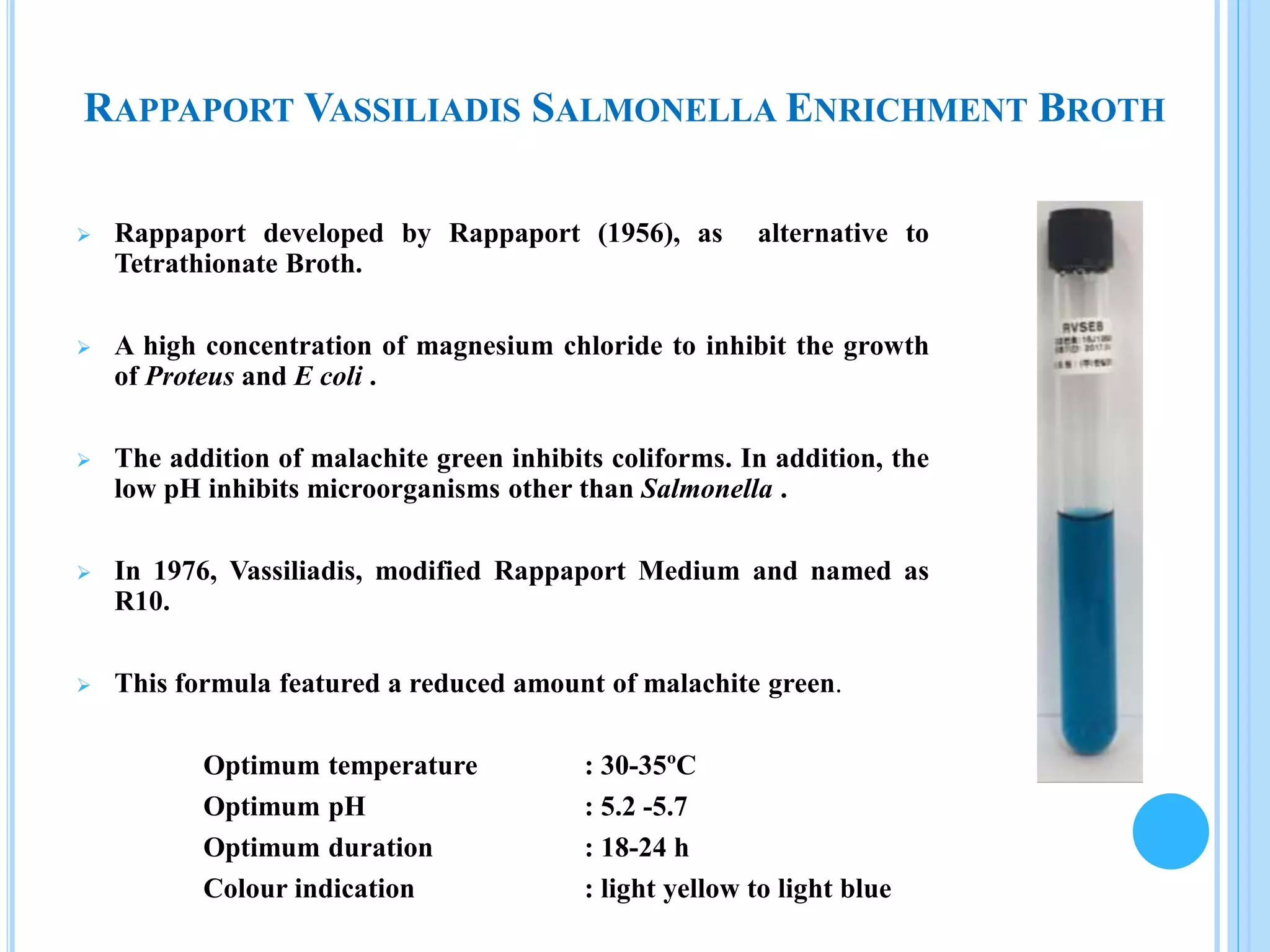
The document outlines microbial limit tests aimed at quantifying viable microorganisms in pharmaceuticals, highlighting issues such as contamination of products with harmful bacteria. It details various methods for microbial detection, such as membrane filtration, pour plate, and selective media suited for specific bacteria like Staphylococcus, Pseudomonas, E. coli, and Salmonella. The document also specifies incubation conditions and indicators for assessing microbial growth in laboratory settings.