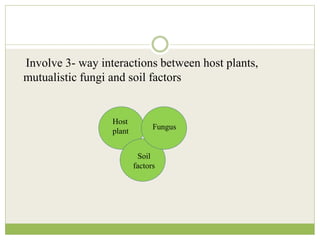
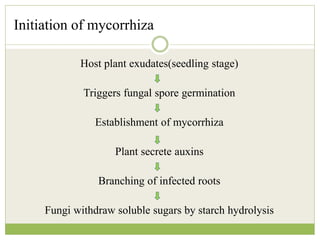
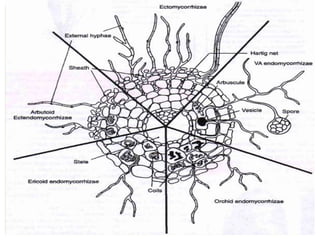
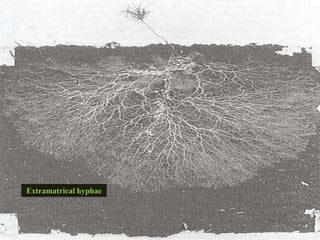
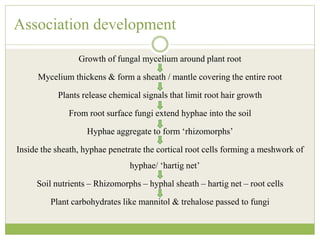
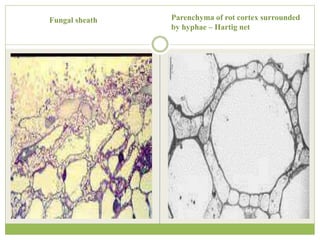
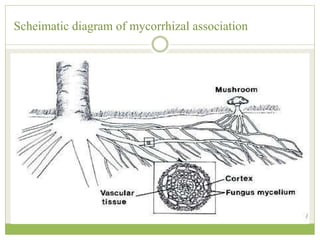
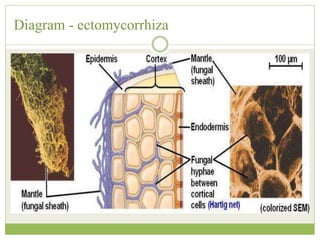
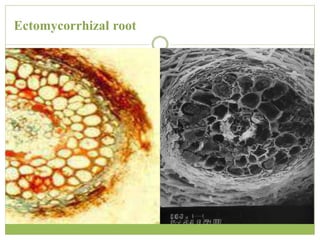
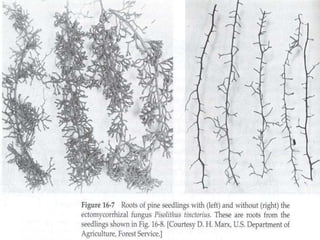
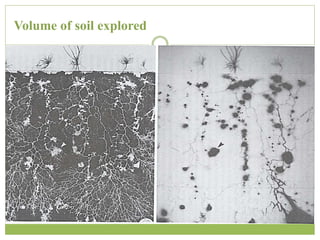
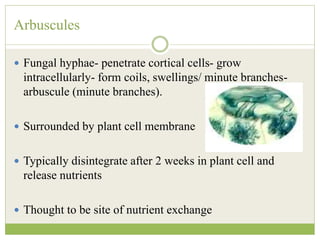
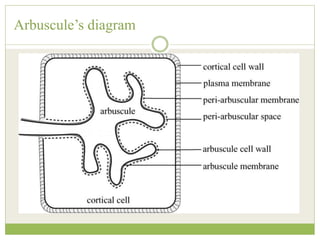
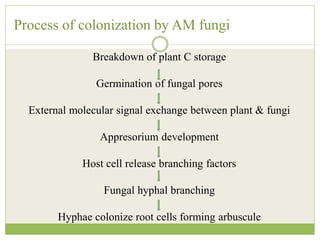
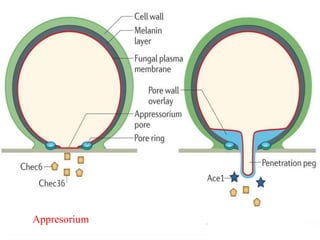
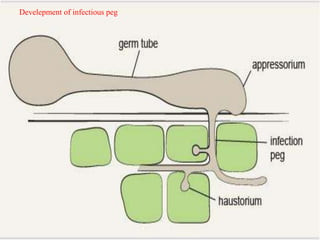
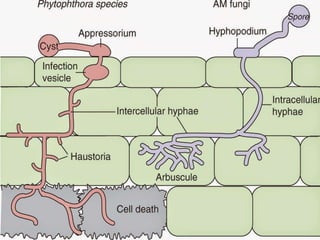
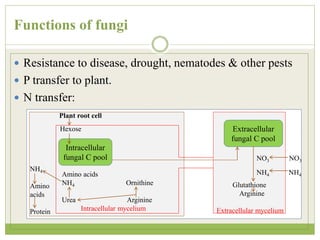
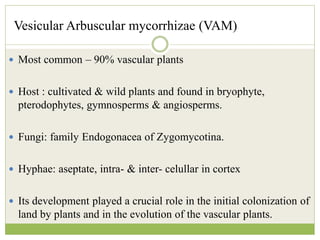
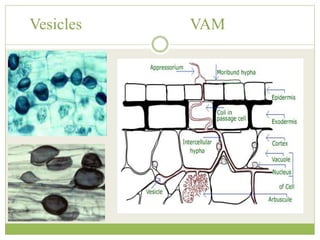
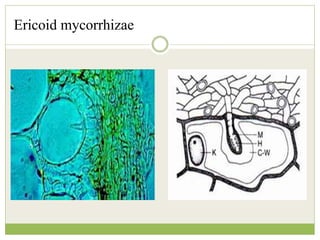
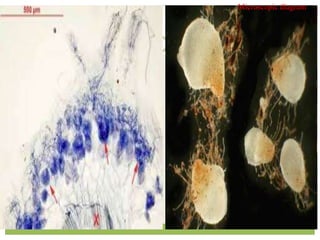
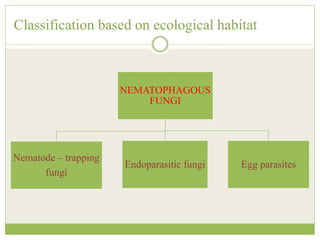
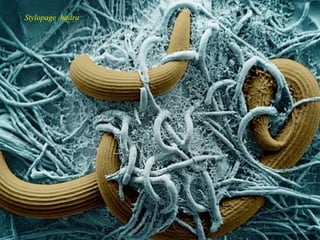
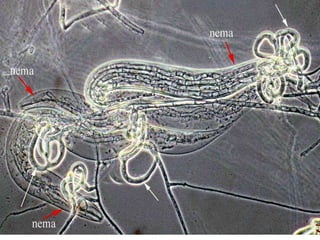
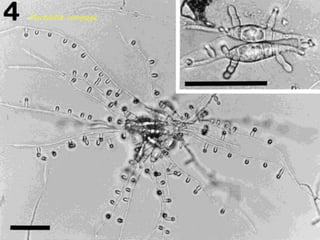
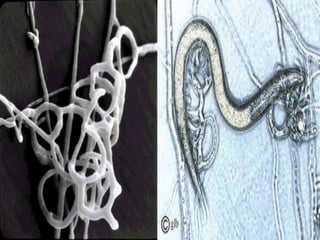
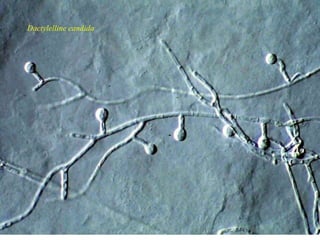
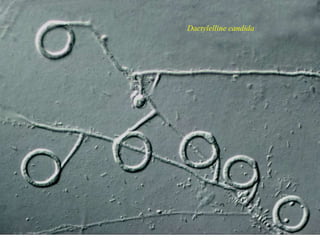
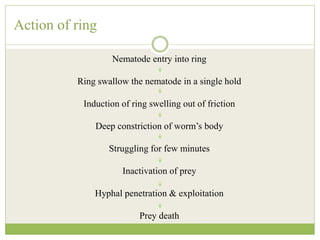
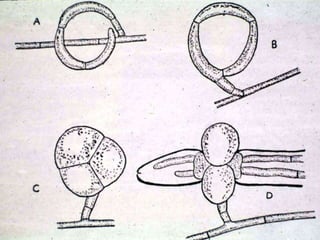
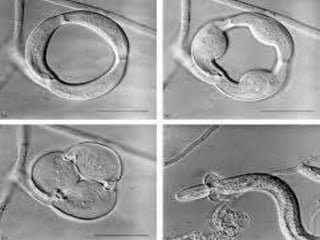
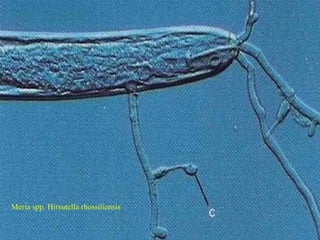
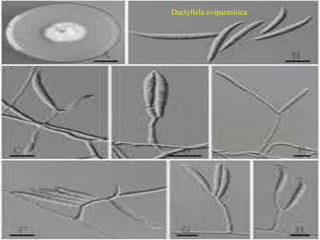
This document discusses mycorrhizal fungi and nematophagous fungi. It begins by introducing mycorrhizae as a symbiotic relationship between fungi and plant roots. It then describes different types of mycorrhizal associations like ectomycorrhizae, endomycorrhizae, and arbuscular mycorrhizae. It also discusses the benefits of mycorrhizal relationships for both plants and fungi. The document then introduces nematodes and nematophagous fungi, which prey on nematodes through trapping mechanisms like adhesive hyphae or nets.