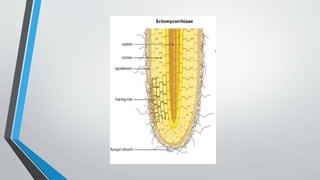
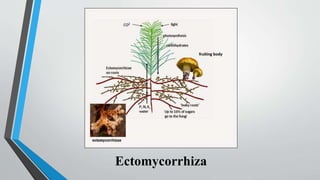
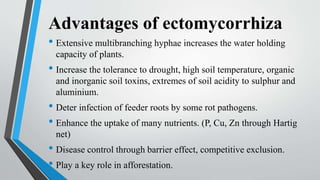
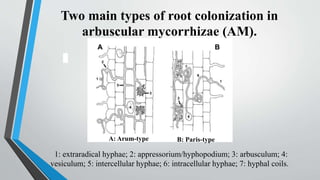
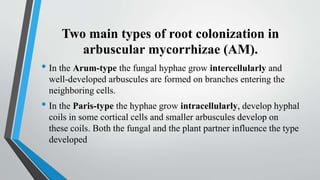
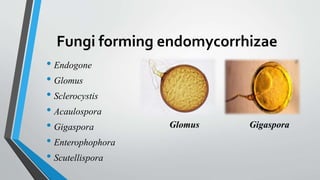
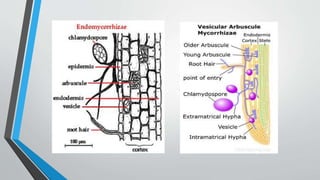
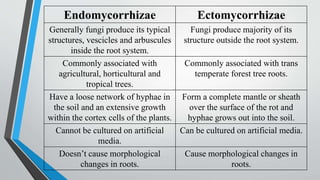
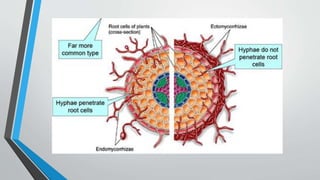
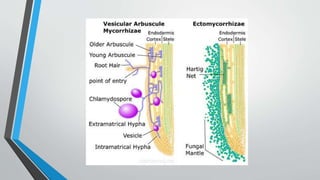
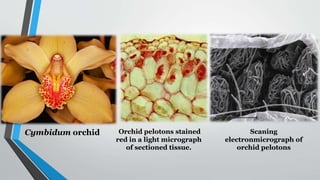
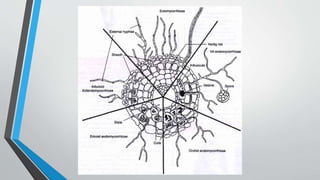
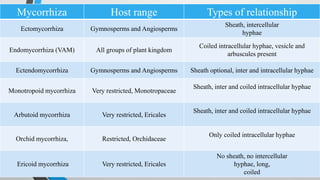
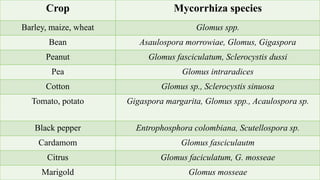
This document provides an overview of mycorrhiza, which is a symbiotic relationship between fungi and plant roots. It defines mycorrhiza and explains that 95% of plant species form these relationships. It then classifies and describes the main types of mycorrhizal associations like ectomycorrhiza, endomycorrhiza, and orchid mycorrhiza. The document outlines the importance and benefits of mycorrhizal relationships for plant growth and health. It also discusses methods for isolating, mass producing, and applying mycorrhizal fungi.