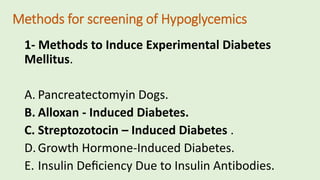
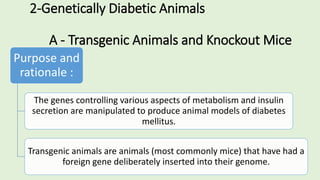
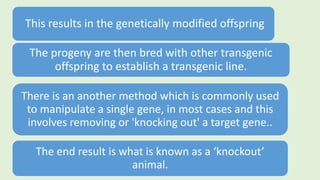
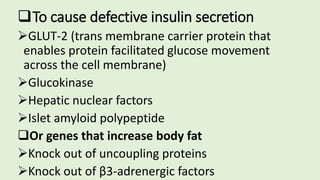
The document discusses the pathophysiology, classification, and current treatment modalities for diabetes, categorizing it into Type 1 and Type 2. It covers various drug screening methods for antidiabetic agents, including experimental induction of diabetes in animal models and measurements of blood glucose-lowering activity. Additionally, it emphasizes the importance of understanding insulin resistance and the impact of specific genes on diabetes through transgenic and knockout animal models.
![•Diabetes is a chronic metabolic disorder characterized
by either the insufficient production or the lack of
response to a key regulatory hormone of the body’s
metabolism, insulin.
•It can be categorized as Type-1 diabetes [insulin
dependent diabetes mellitus (IDDM)] and Type-2
diabetes [non- insulin dependent diabetes mellitus
(NIDDM)].
•The overall prevalence of diabetes is approximately 10%
of the population, of which 90% is Type-2.](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-2-320.jpg)
















![•2-deoxy-2-[3-methyl-3-nitrosourea] 1-D
glucopyranose
•Initially used for antibacterial & anti-tumor
activity
•From fungus Streptomyces achromogenes
Induces permanent DM :
By methylation
Free radical generation
Nitric oxide production](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-23-320.jpg)









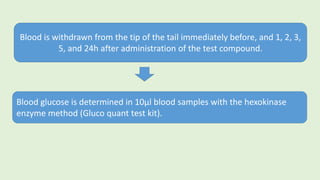



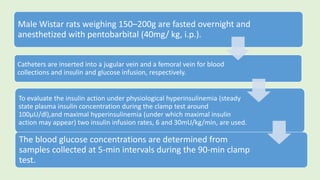

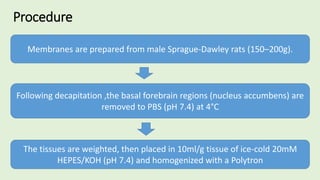
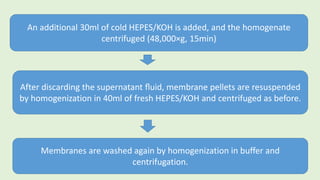
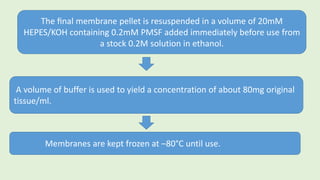
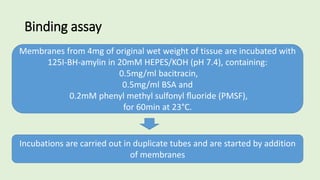


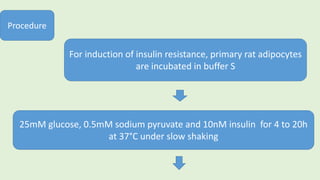



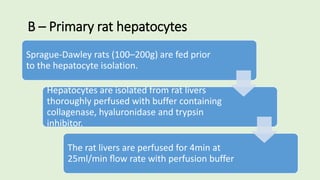
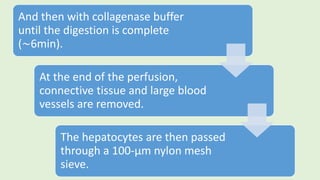



![The cells are lysed and the total lipids separated from water-
soluble products and the incubation medium including the
unincorporated[3H]glucose by addition of toluene-based
scintillation cocktail.
For measurement of lipogenesis monitoring effects on both
glucose transport and esterification, isolated rat adipocytes are
incubated with D-[3H]glucose (0.55mM final concentration,0.1–
1µCi).
Procedure](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-59-320.jpg)
![The reaction is started by the transfer of 0.2ml of adipocyte suspension (3.5×105
cells/ml) in KRHB to scintillation vials containing 0.1ml of [3-3H] glucose
(2µCi/ml,4.4mM),0.4 ml of 2-fold KRHB and 0.3ml of insulin or compound/drug with
insulin-mimetic activity dissolved in vehicle (e.g. DMSO) and diluted with KRHB to the
appropriate concentration of compound and vehicle (e.g. 3% DMSO).
After phase separation, radioactivity incorporated in to total lipids/phospholipids is
determined by liquid scintillation counting directly without removal of the lipid
phase based on determination of the radiolabel of the lipidic products partitioned
into the toluene phase containing the scintillator rather than of the [3H]glucose
left in the aqueous phase lacking scintillator.](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-60-320.jpg)
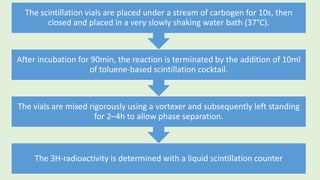
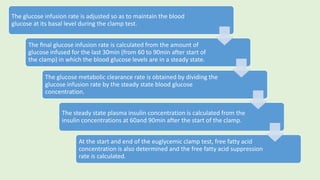
![Evaluation
The blank values lacking adipocytes (usually500–600dpm) are subtracted from
the values measured for the corresponding set of test mixtures containing
adipocytes to correct for 3H-radiation originating from the aqueous phase (i.e.
[3H]glucose left in the incubation medium).
Fold stimulations reflecting the responsiveness of the glucose transport
and/or esterification systems of the adipocytes toward
insulin/compound/drug are calculated as ratio between the corrected test
values (presence of insulin/compound/drug)and basal values (absence of
insulin/compound/drug).
Typically, insulin induces 15to 20-fold, 8- to 12-fold and 2.5- to 4-fold
stimulations in lipogenesis at 50µM, 0.55mM and 2mM glucose, respectively.](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-62-320.jpg)



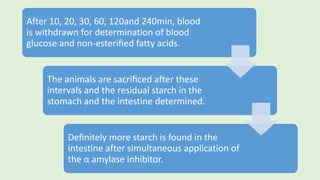


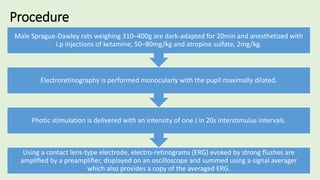
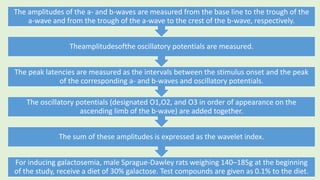

![PROCEDURE
The initial steps are performed in vitro, focused on receptor interaction.
Subsequent steps in-vitro to explore the receptor-mediated
signaling, which is however at the present time difficult to attribute
precisely to biological and clinical effects.
The main aim of the in vitro evaluation is to establish the relation of
metabolic activity to mitogenic activity, as previously done for structure
activity studies on insulin analogs, and subsequently directed to predicting
clinical relevance of these observations, when comparing new compounds
with the established biochemical and toxicological profile of [B10-Asp]
insulin, and the clinically used fast acting and long acting insulin analogs.](https://image.slidesharecdn.com/methodsforscreeningofhypoglycemics-180928062900/85/Methods-for-screening-of-hypoglycemics-73-320.jpg)
