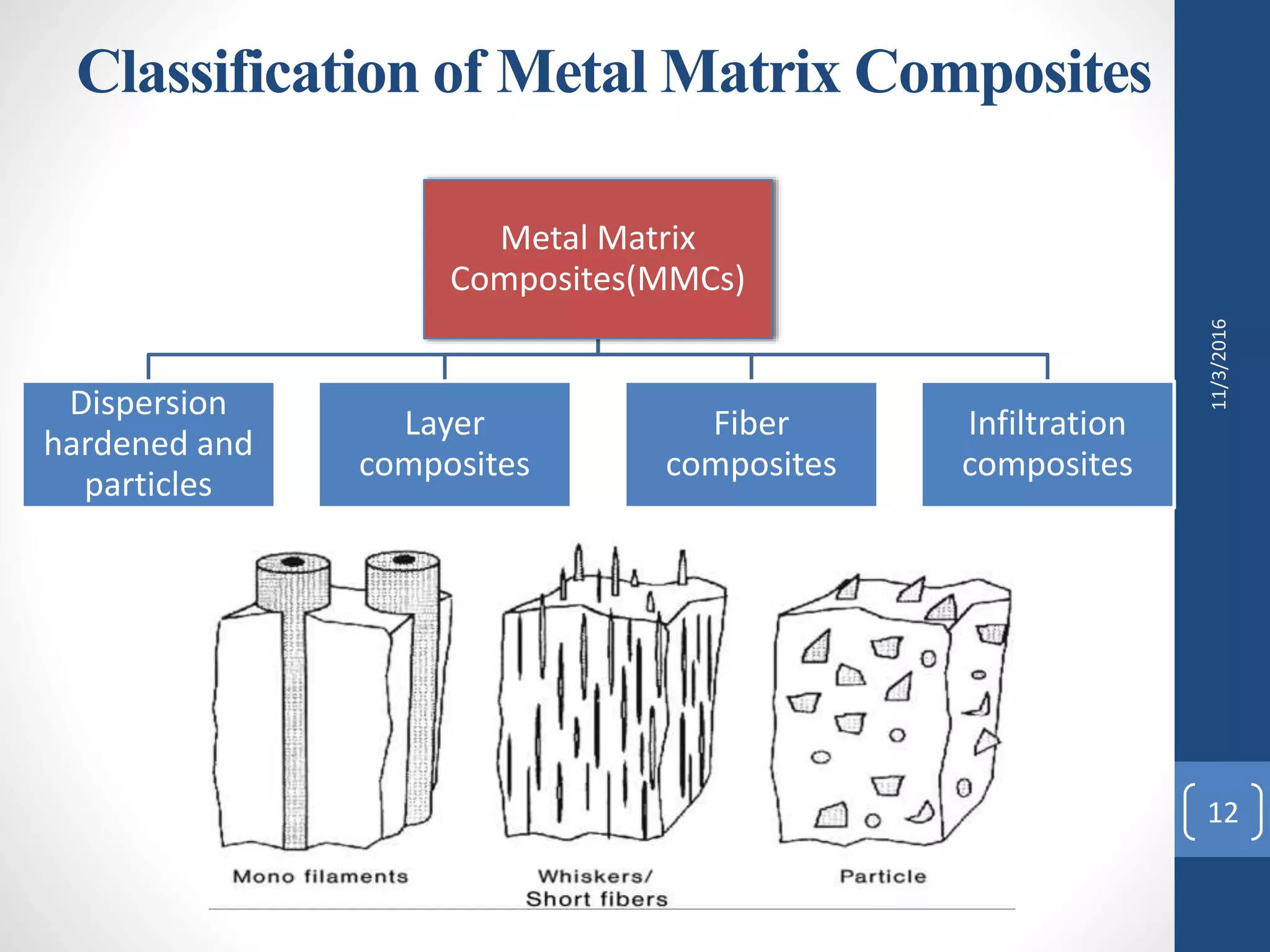
The document provides an overview of metal matrix composites (MMCs). It discusses that MMCs consist of a metal matrix reinforced with ceramic particles or fibers. The reinforcement improves the composite's properties over the unreinforced metal, such as increased strength and stiffness. The document also examines the important interfaces between the matrix and reinforcement, which influence the composite's performance. It describes various bonding mechanisms at the interface like mechanical, chemical, and diffusion bonding. Finally, the document outlines common processing techniques for fabricating MMCs, including powder metallurgy where metal powders are compacted and sintered to form the final composite material.