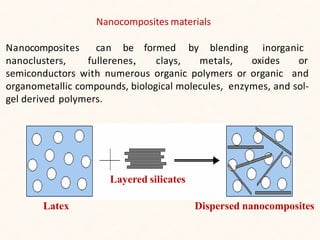
This document discusses metal matrix nanocomposites. It defines nanocomposites as consisting of two phases, with one being nanosized particles embedded in a matrix material. Metal matrix nanocomposites (MMNCs) specifically use a metal as the matrix and a ceramic as the reinforcement. Carbon nanotube metal matrix nanocomposites are also discussed. The document outlines various synthesis routes for fabricating MMNCs, including solid-state and liquid-state processing methods, and discusses some advantages and limitations of different processing techniques. Properties of MMNCs include increased hardness, strength, and superplasticity as well as lowered melting point and improved electrical and magnetic properties.