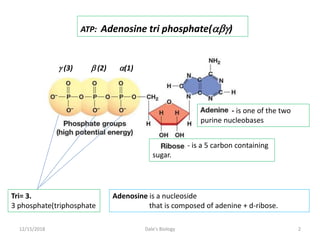
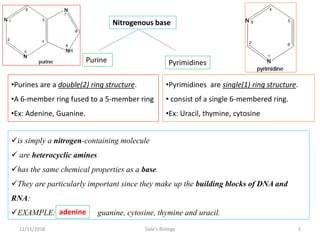
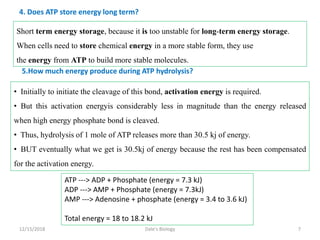
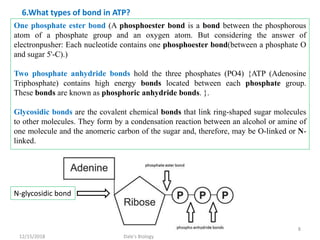
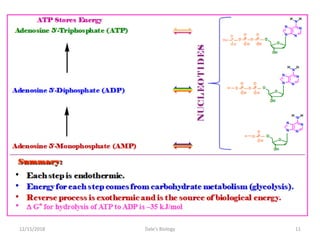
1. Adenosine triphosphate (ATP) is composed of adenine, ribose sugar, and three phosphate groups. It acts as the main energy currency in cells.
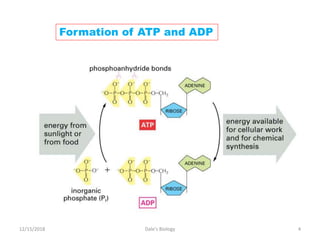
2. ATP stores and transports chemical energy within cells by breaking its high-energy phosphate bonds. When a cell needs energy, it hydrolyzes ATP to ADP, releasing energy. Excess energy is stored by reforming ATP from ADP and phosphate.
3. ATP is uniquely suited to act as the primary energy carrier in cells. Its high-energy bonds are unstable and readily broken to fuel cellular reactions, then reformed when energy is available. This allows ATP to efficiently provide short-term energy storage and transport.