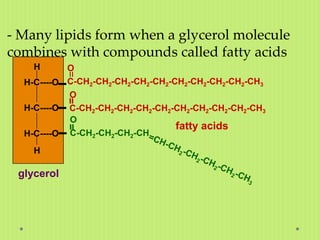
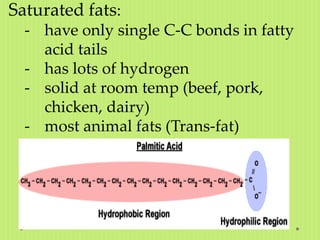
Lipids are a diverse group of hydrophobic biomolecules that include fats, oils, waxes, phospholipids and steroids. They are made up of fatty acid chains bonded to a glycerol molecule or other alcohol. Lipids serve important functions like energy storage, insulation of nerve fibers, and as structural components of cell membranes. The main classes of lipids are triglycerides, phospholipids, and steroids; triglycerides are the main form of fat storage in animals and are composed of fatty acid chains bonded to a glycerol molecule.