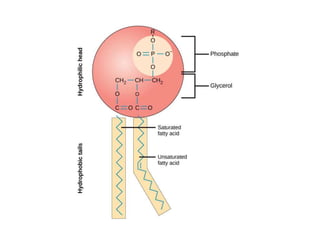
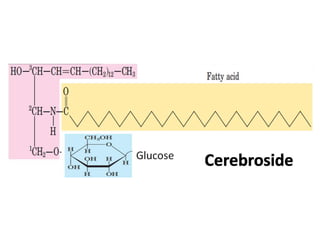
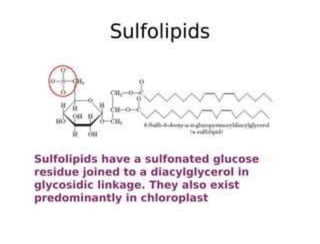
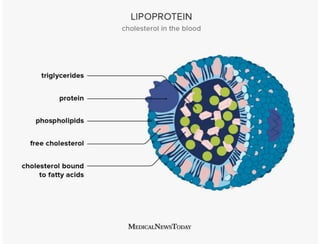
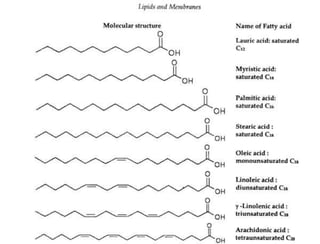
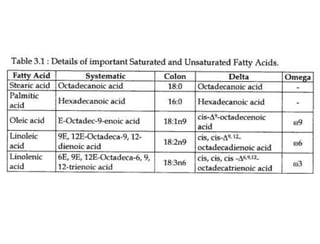
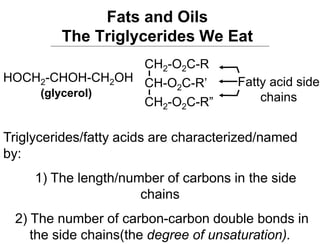
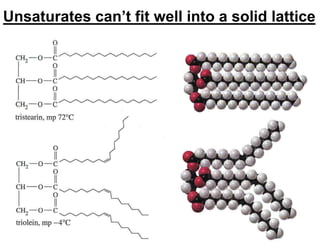
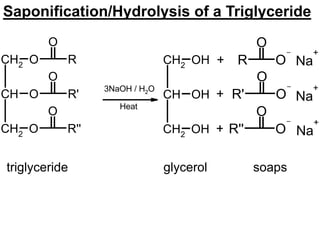
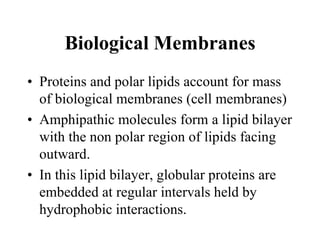
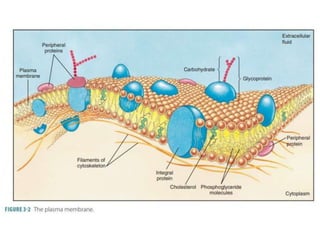
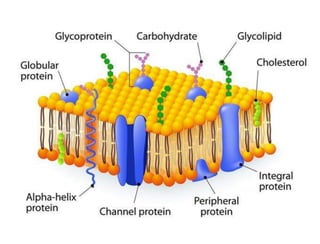
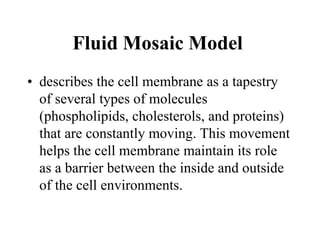
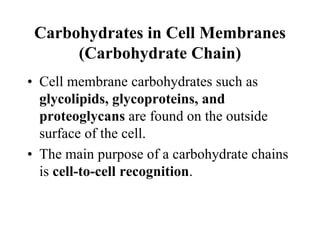
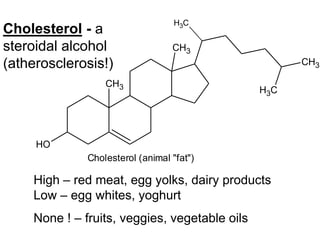
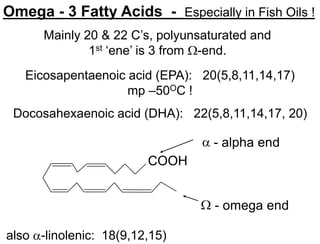
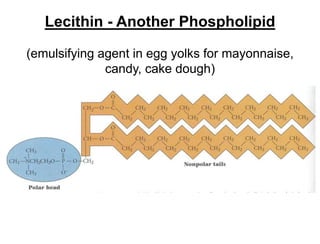
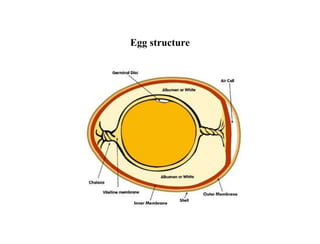
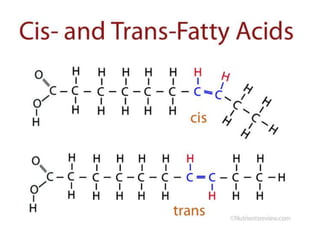
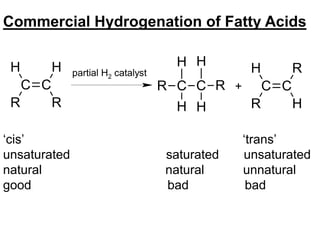
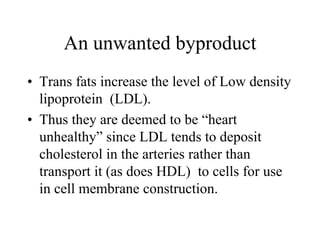
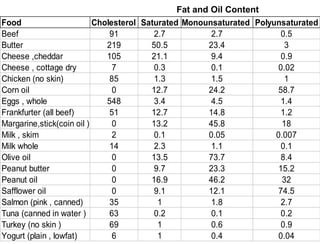
Lipids include fats, oils, waxes, and steroids. They are insoluble in water but soluble in organic solvents. Fats and oils make up 95% of nutritional lipids and occur as both storage and structural components in plants and animals. Lipids play important roles including providing palatability to foods, supplying essential fatty acids, and aiding in vitamin absorption. They are classified based on their structure as simple lipids like fats/oils, compound lipids containing additional groups, or derived lipids formed from hydrolysis. Biological membranes contain lipids that form a fluid bilayer, maintaining permeability and hosting embedded proteins. Membranes are essential for cellular structure and function.