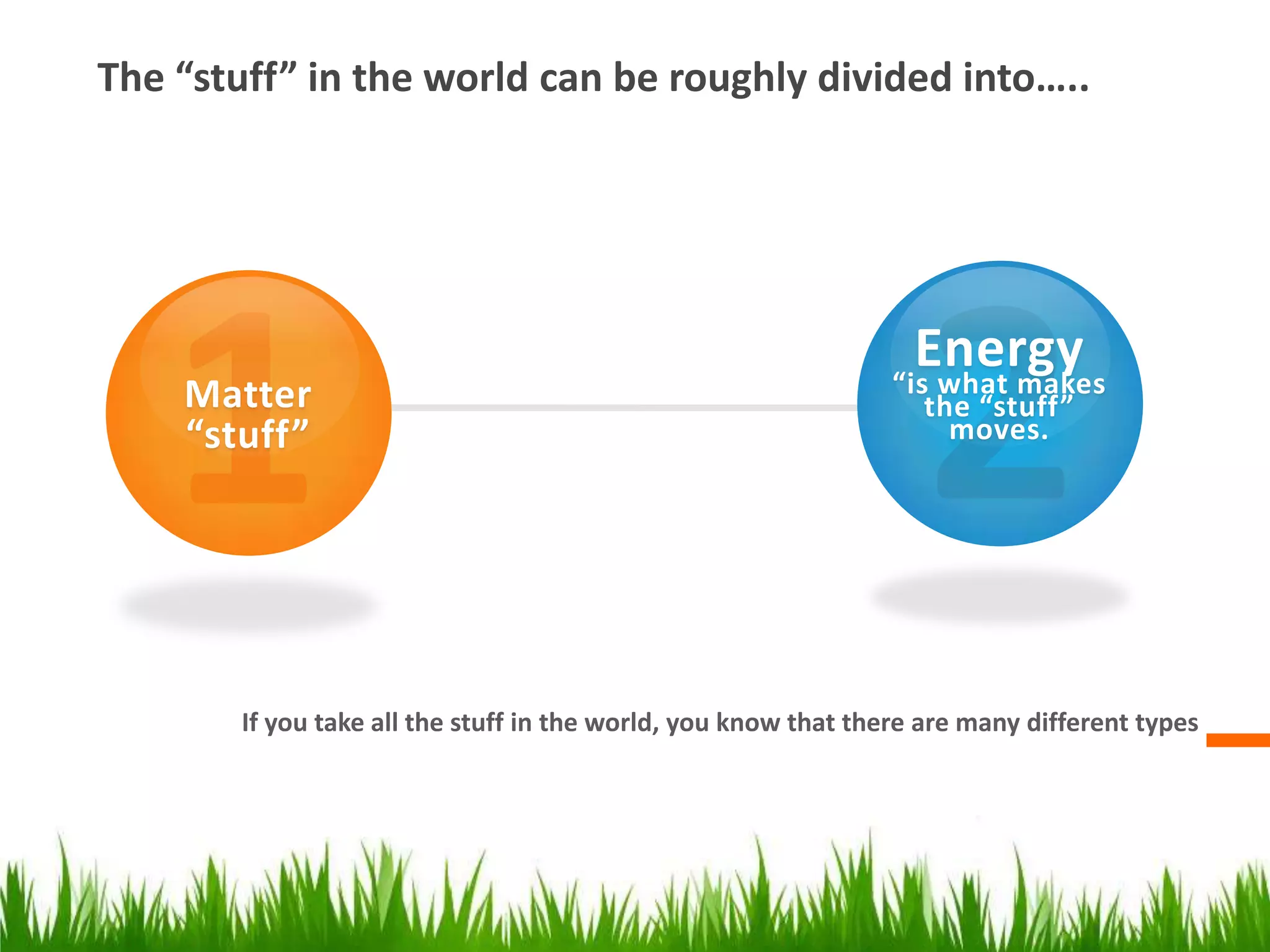
The document discusses the key concepts of matter and its properties. It defines matter as anything that has mass and takes up space. Matter can be found in three states - solid, liquid, and gas - and has various properties that can be measured like density, volume, and temperature. It also discusses concepts like conductivity, magnetism, and solubility as they relate to different types of matter. Conductors and insulators are contrasted in terms of their ability to allow electricity and heat to flow through them. Overall, the document provides an overview of the fundamental properties and types of matter through definitions, examples, and discussion of key terms and concepts.