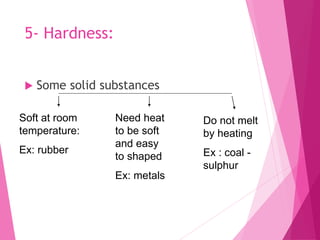
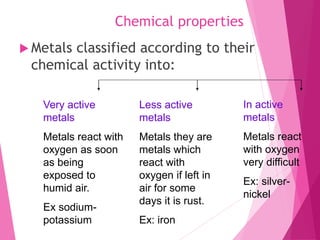
Matter is anything that has mass and occupies space. It can be distinguished by physical properties like color, density, melting point, boiling point, hardness, and conductivity, or by chemical properties like reactivity. Density is a physical property defined as mass per unit volume. It allows materials to be differentiated as some materials sink in water while others float, and is important in applications like balloons and determining purity. Melting and boiling points are temperatures at which matter changes state, and vary between materials. Hardness, conductivity, and other properties influence what materials are suitable for different uses like cookware or wires. Chemical reactivity ranges from very reactive metals like sodium to less reactive like iron to inactive like silver.