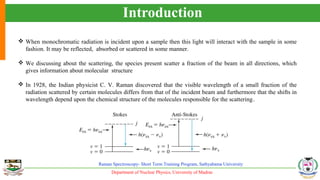
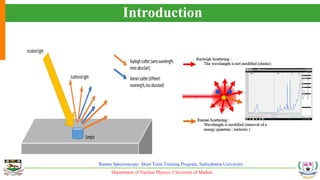
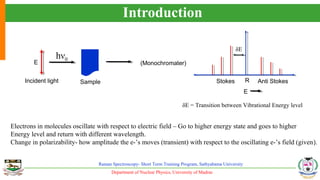
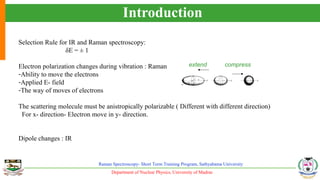
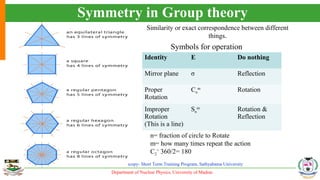
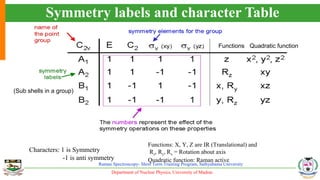
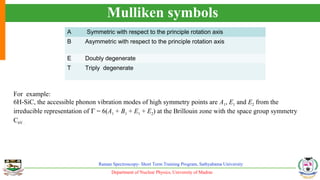
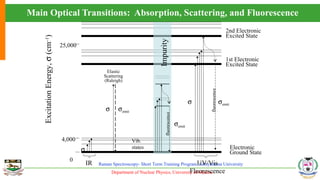
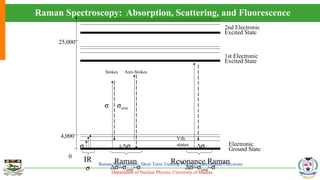
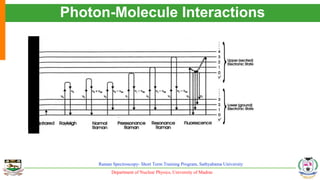
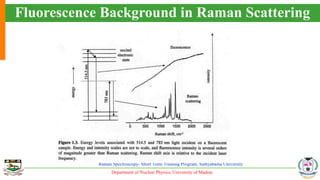
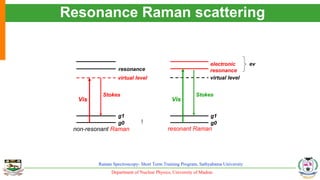
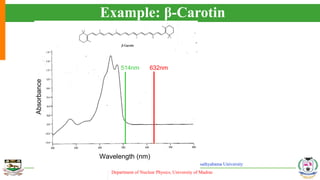
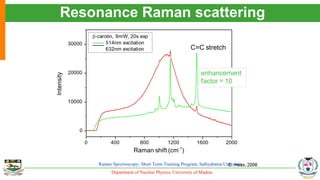
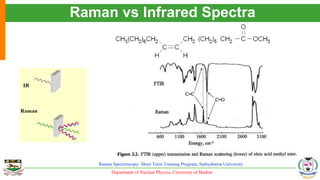
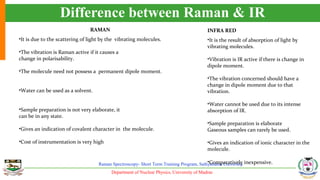
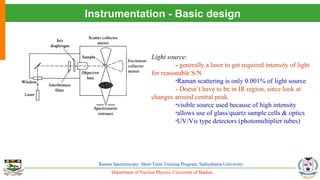
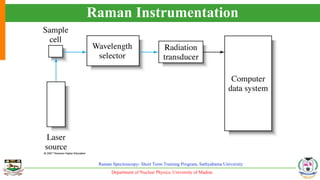
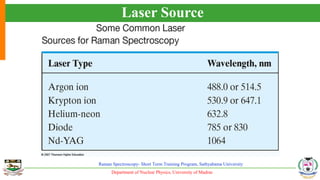
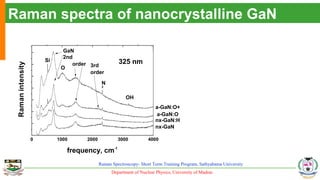
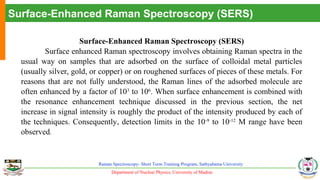
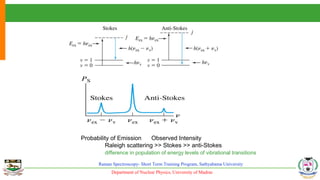
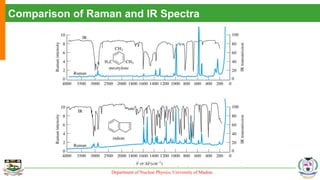
The document provides an overview of Raman spectroscopy, outlining its principles based on light scattering and molecular vibration, as well as the historical development and significance of the technique. It discusses the differences between Raman and infrared spectroscopy, the instrumentation involved, and applications such as depth profiling and surface-enhanced Raman spectroscopy. Key concepts such as symmetry, character tables, and selection rules are also addressed, emphasizing the technique's advantages for analyzing molecular structures.