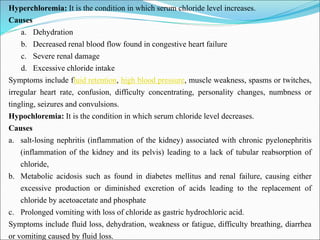
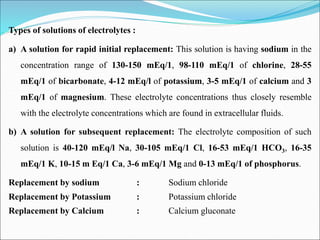
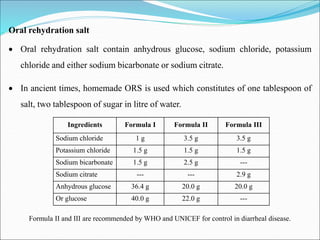
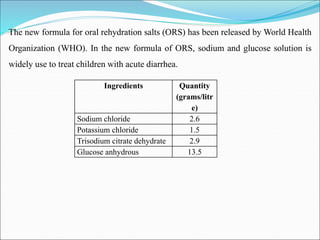
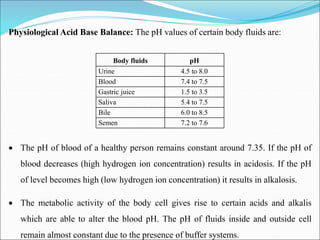
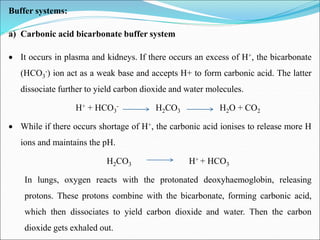
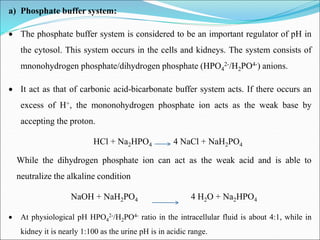
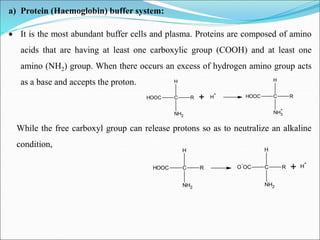
This document summarizes key aspects of electrolytes in the human body. It discusses the different body fluid compartments and their electrolyte concentrations. The major physiological ions (sodium, potassium, calcium, phosphorus, chloride, magnesium) are described along with their functions, normal ranges, and causes of imbalances. Electrolyte replacement therapies and oral rehydration salts are also outlined. Additionally, the document covers acid-base balance and the major buffer systems that help maintain pH homeostasis in the body.