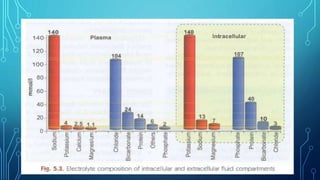
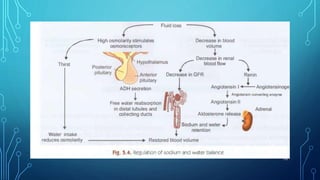
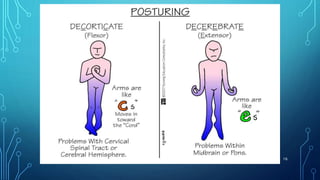
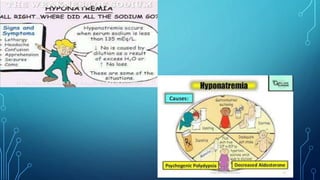
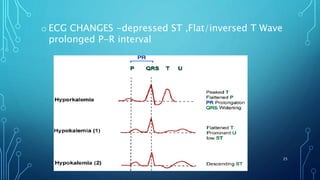
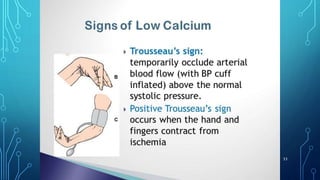
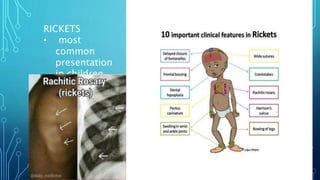
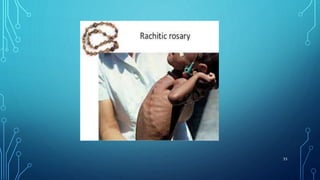
This document provides information on electrolyte disorders presented by Dr. S. Keerthi at J.S.P.S Govt Homoeopathic Medical College. It defines electrolyte disorders as imbalances in ions like sodium, potassium, calcium, magnesium, bicarbonate, phosphate, and chloride. For each electrolyte, the document outlines its normal range, functions, causes of deficiencies and excesses, associated signs and symptoms, and treatment considerations. The goal is to educate on electrolyte regulation and disorders seen in clinical practice.