Leucocyte cytochemistry techniques identify enzymes and substances in blood cells that aid in diagnosis. They are useful for characterizing immature cells in acute myeloid leukemia and identifying abnormalities in myelodysplastic syndromes and myeloproliferative neoplasms. Sudan Black B staining identifies granules in granulocytes, eosinophils, and some monocytes, similar to myeloperoxidase staining. Prussian blue staining identifies non-hem iron in cells, assessing iron stores and availability to developing red blood cells. Proper sample fixation and controls are important for accurate results with these staining techniques.














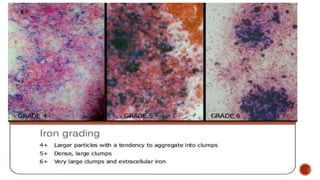
![ The granules (containing ferric iron) react with pottassium ferrocyanide
[K4Fe(CN)6] to form a blue compound ferriferrocynanide), Prussian blue reaction.](https://image.slidesharecdn.com/leucocytecytochemistry-210215195459/85/Leucocyte-Cytochemistry-15-320.jpg)