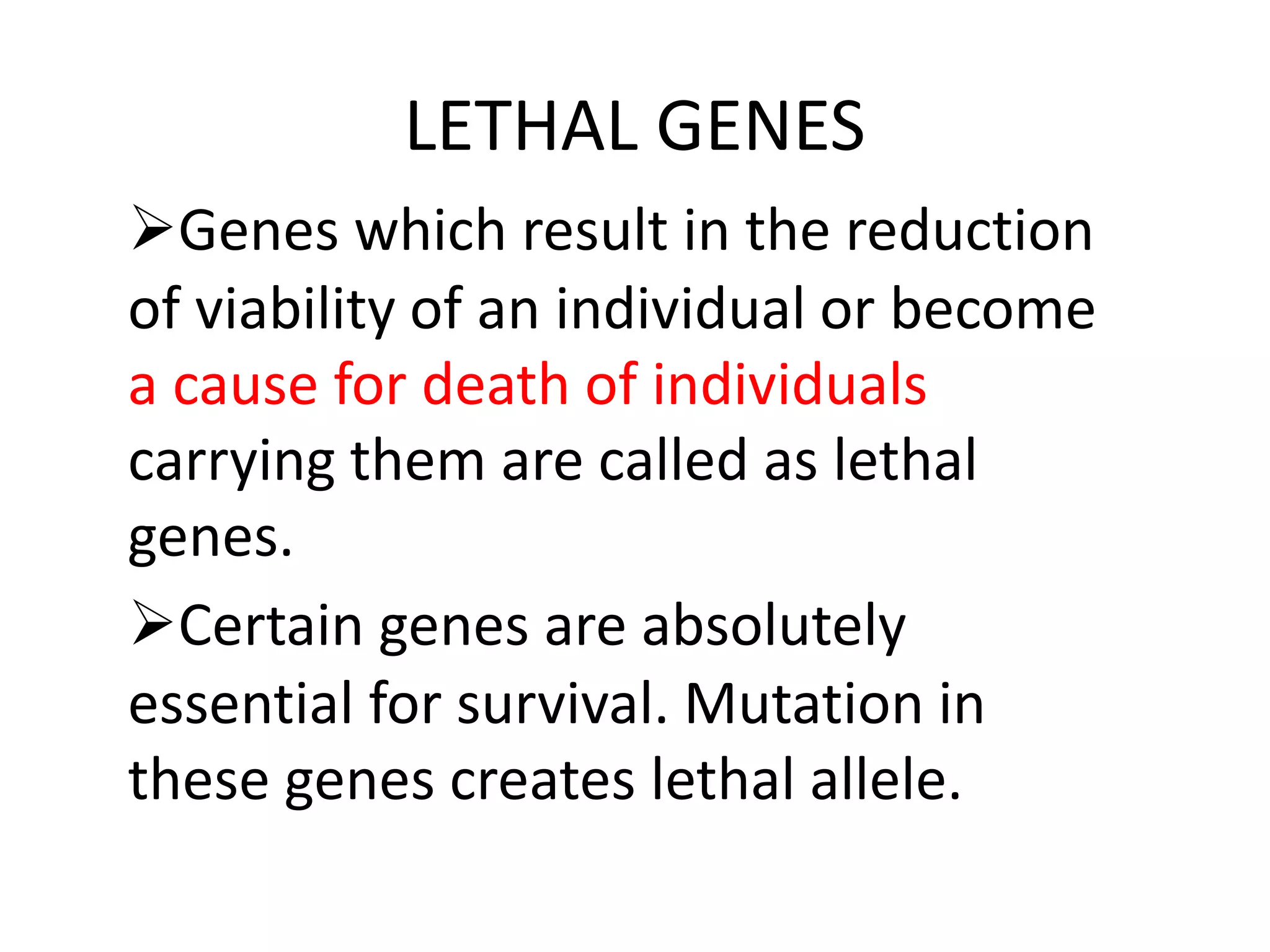
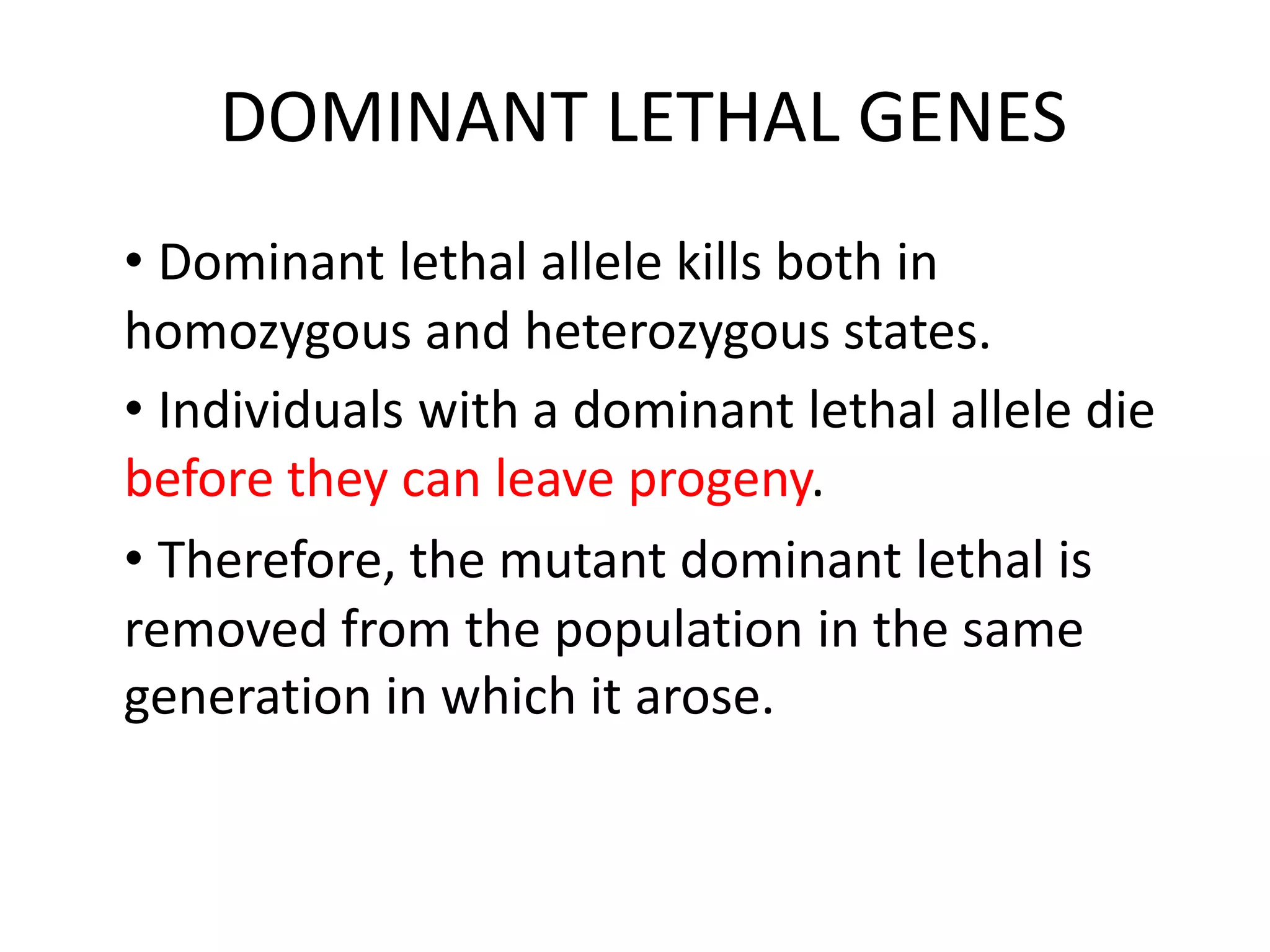
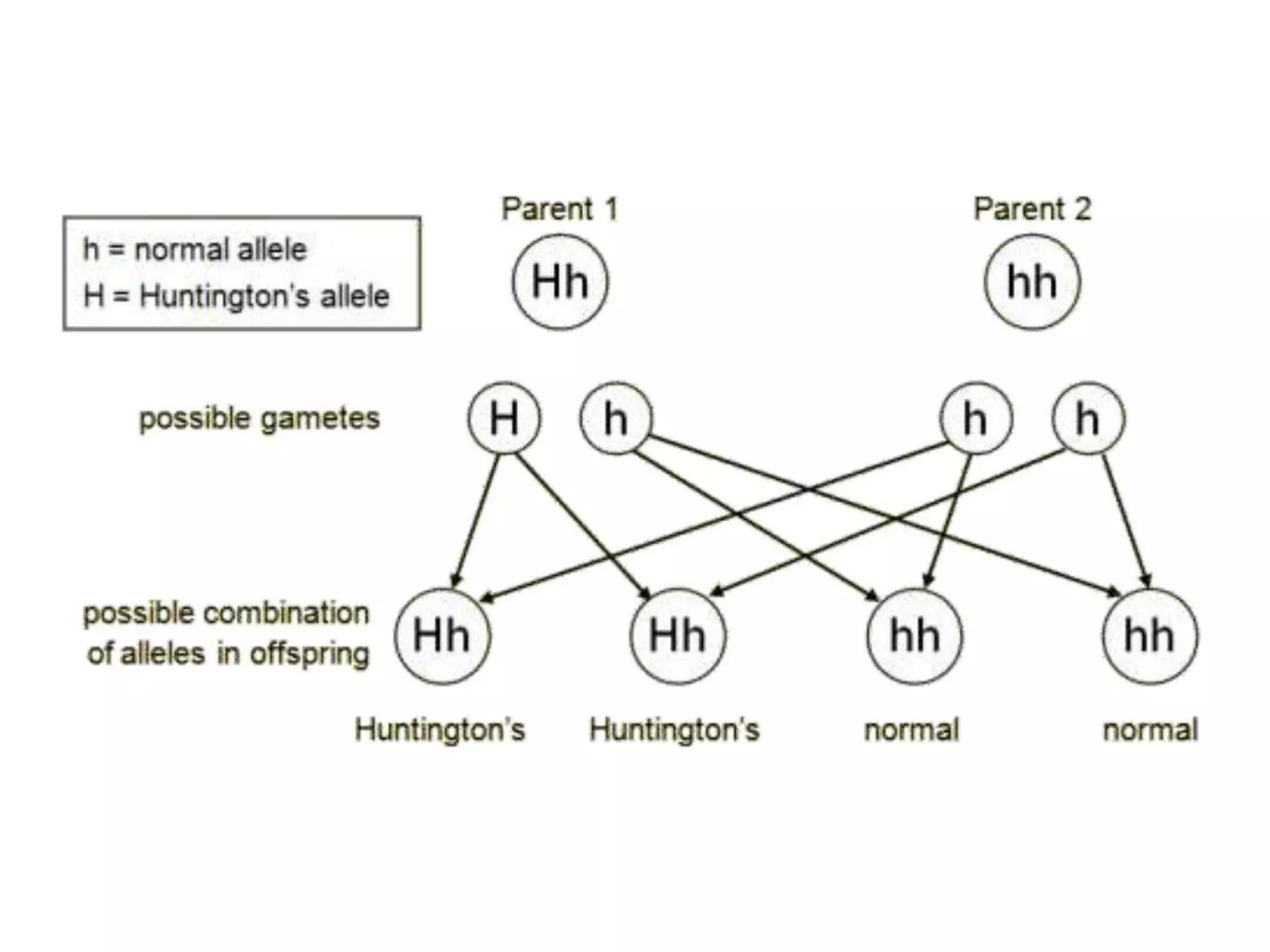
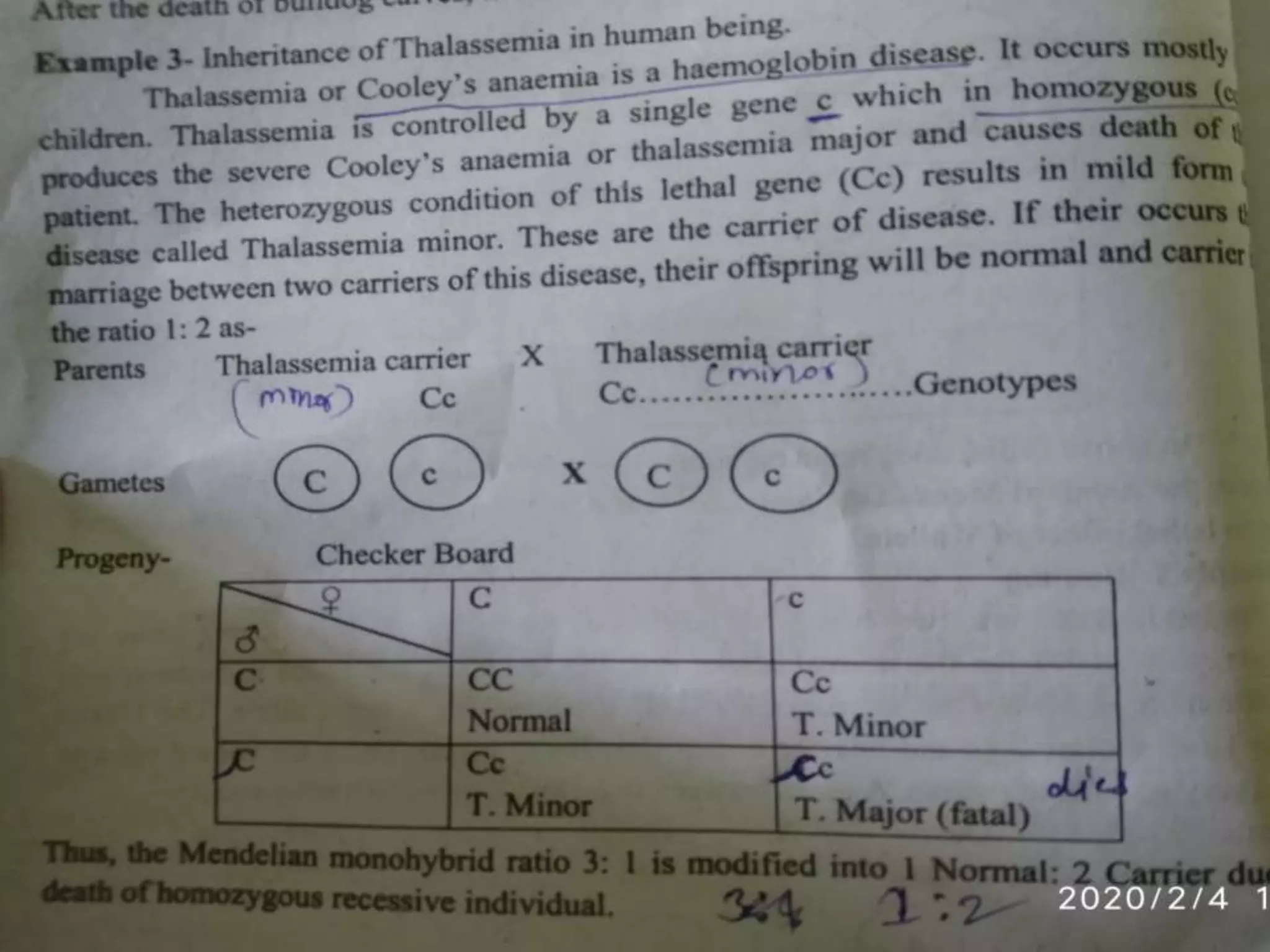
1. Lethal genes are genes that cause death or reduced viability when carried by an individual. They can be dominant or recessive.
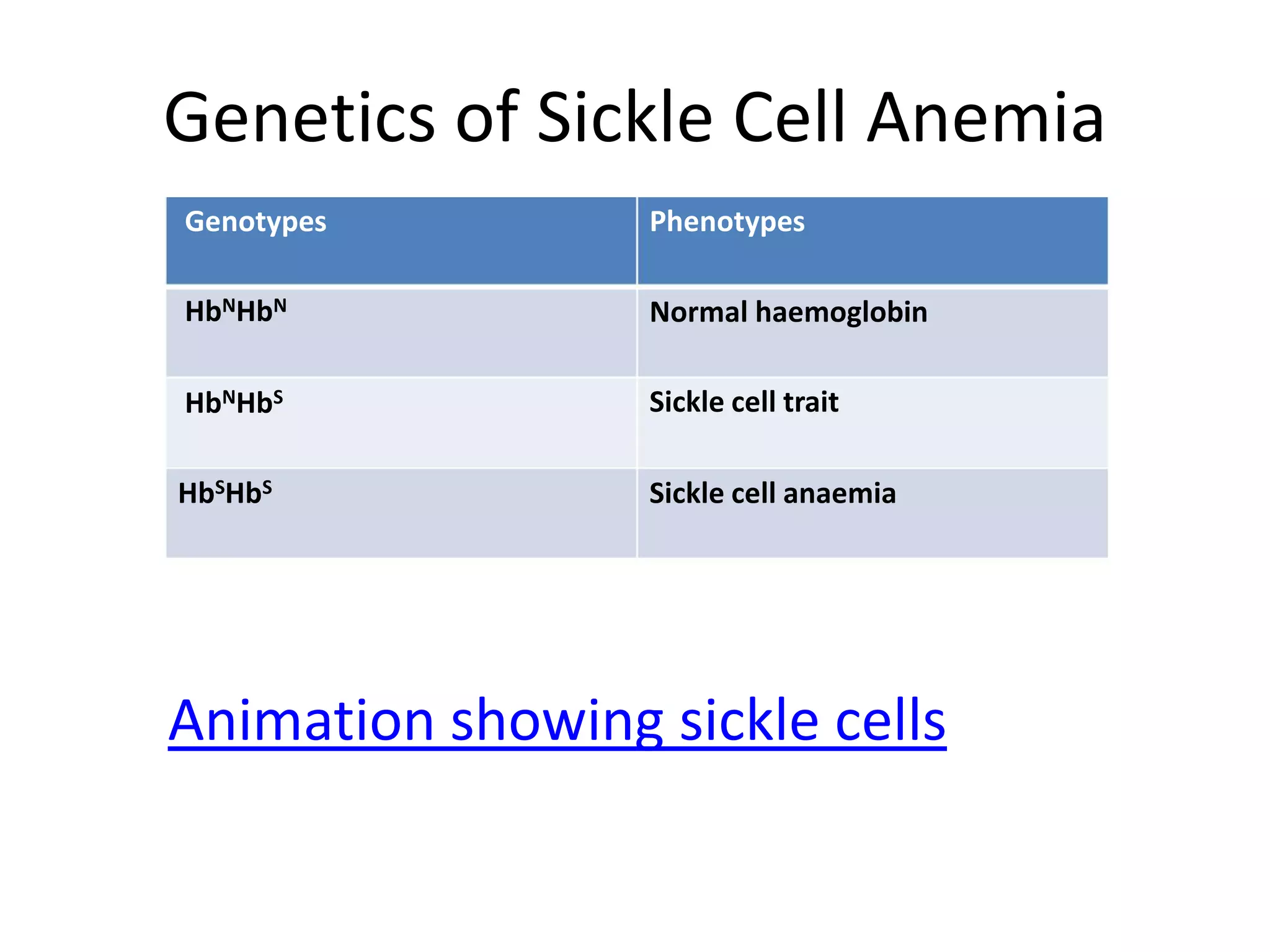
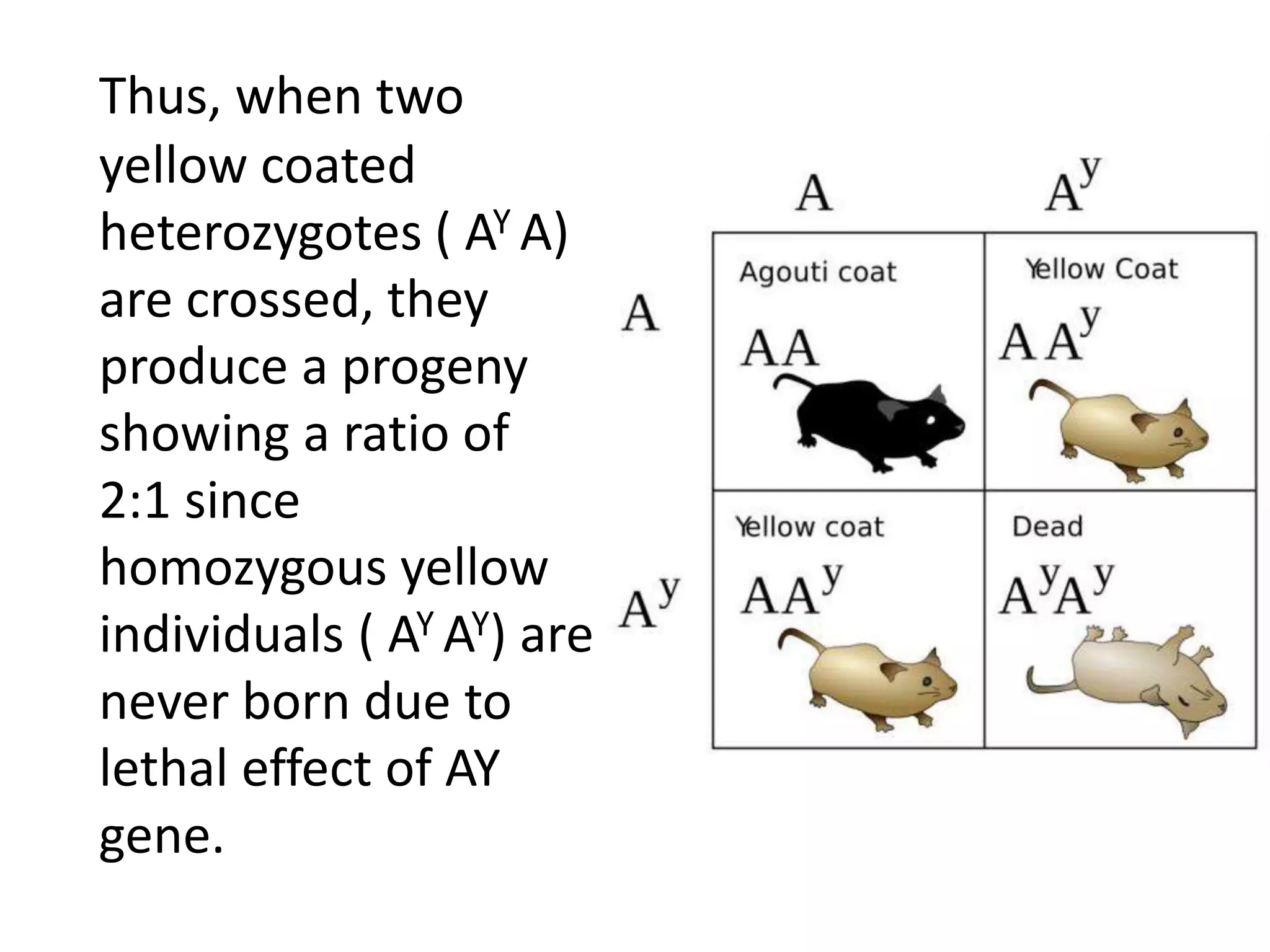
2. Some examples of lethal genes discussed are Huntington's disease, cystic fibrosis, sickle cell anemia, and coat color genes in mice.
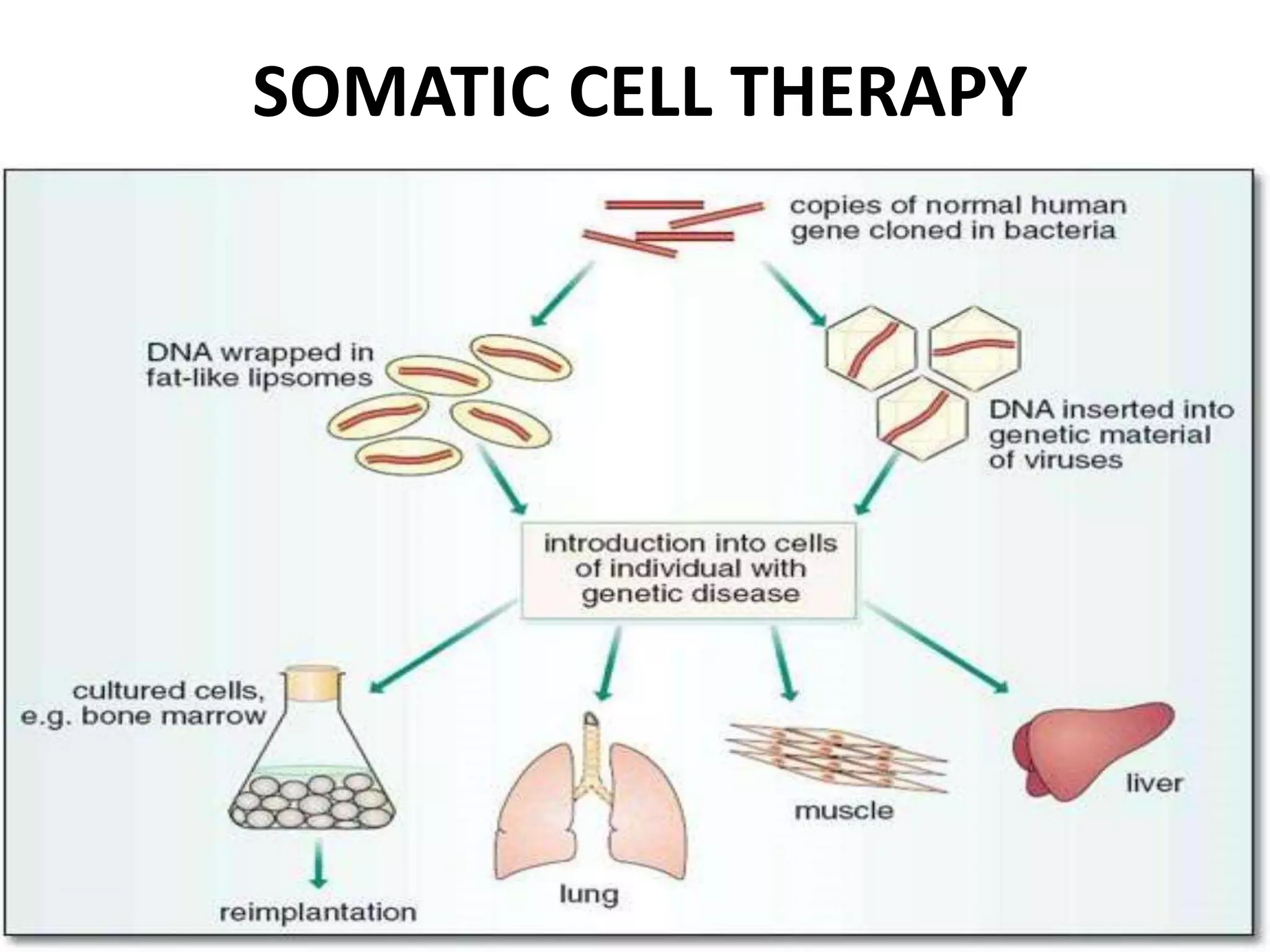
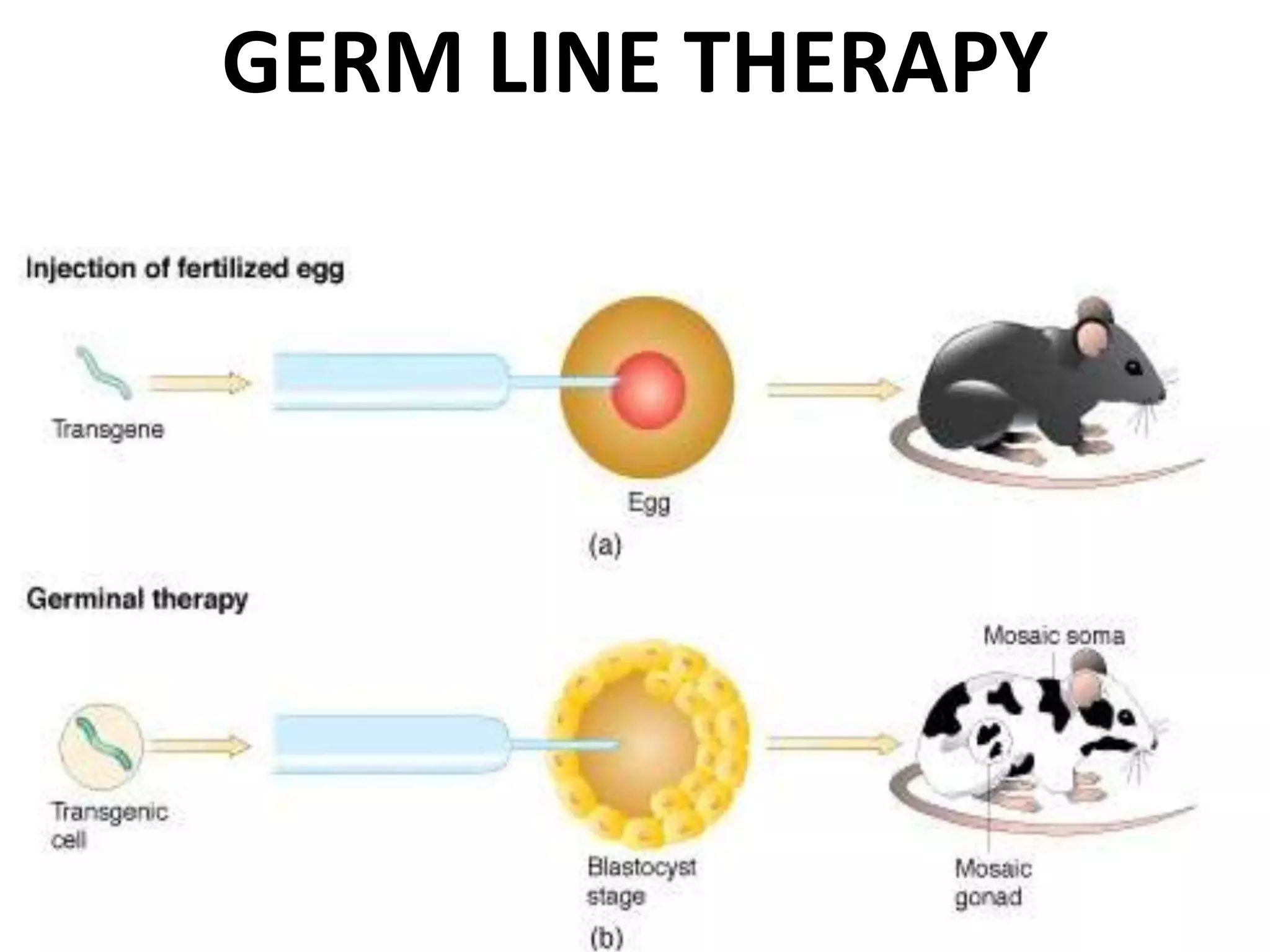
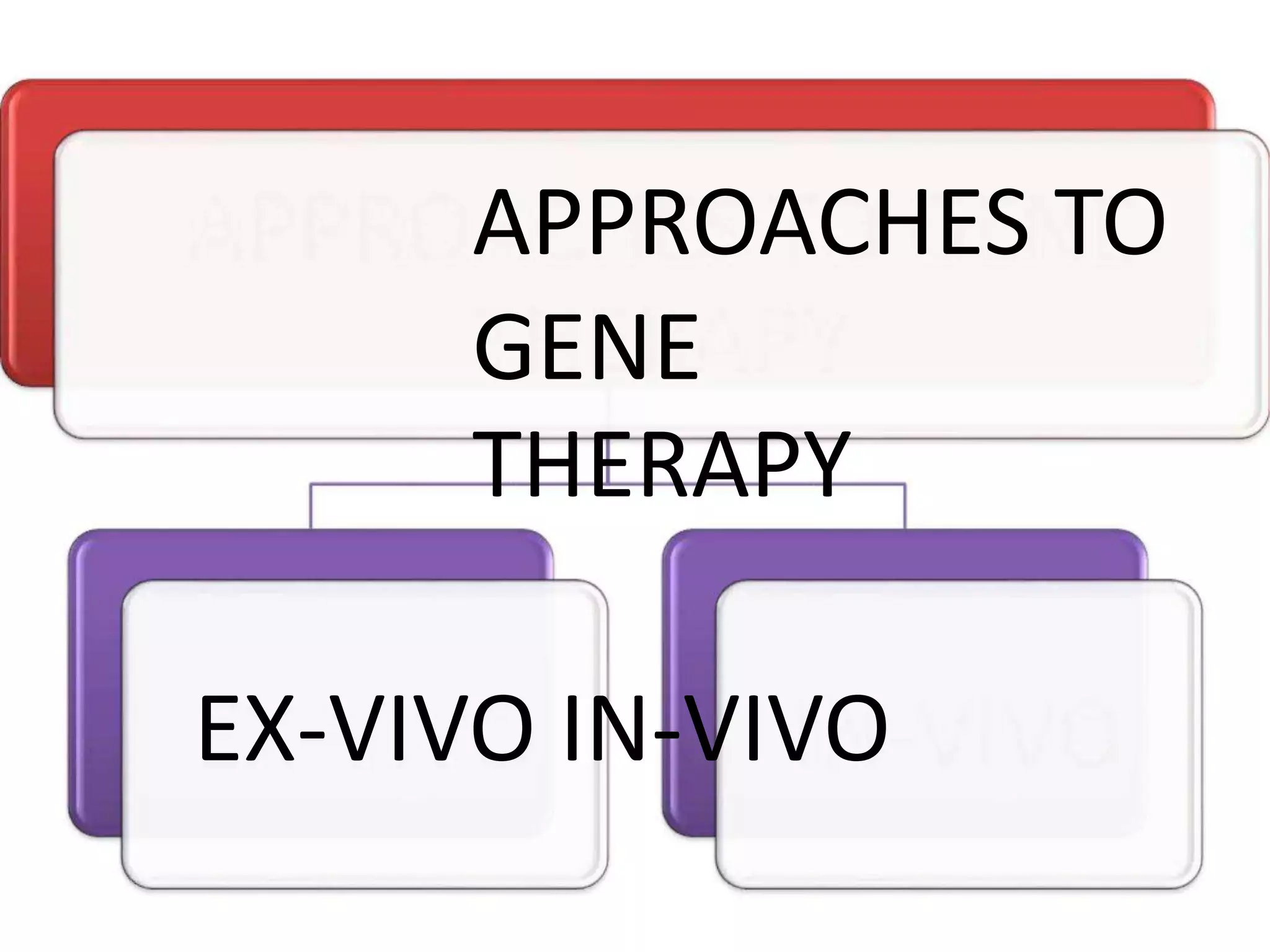
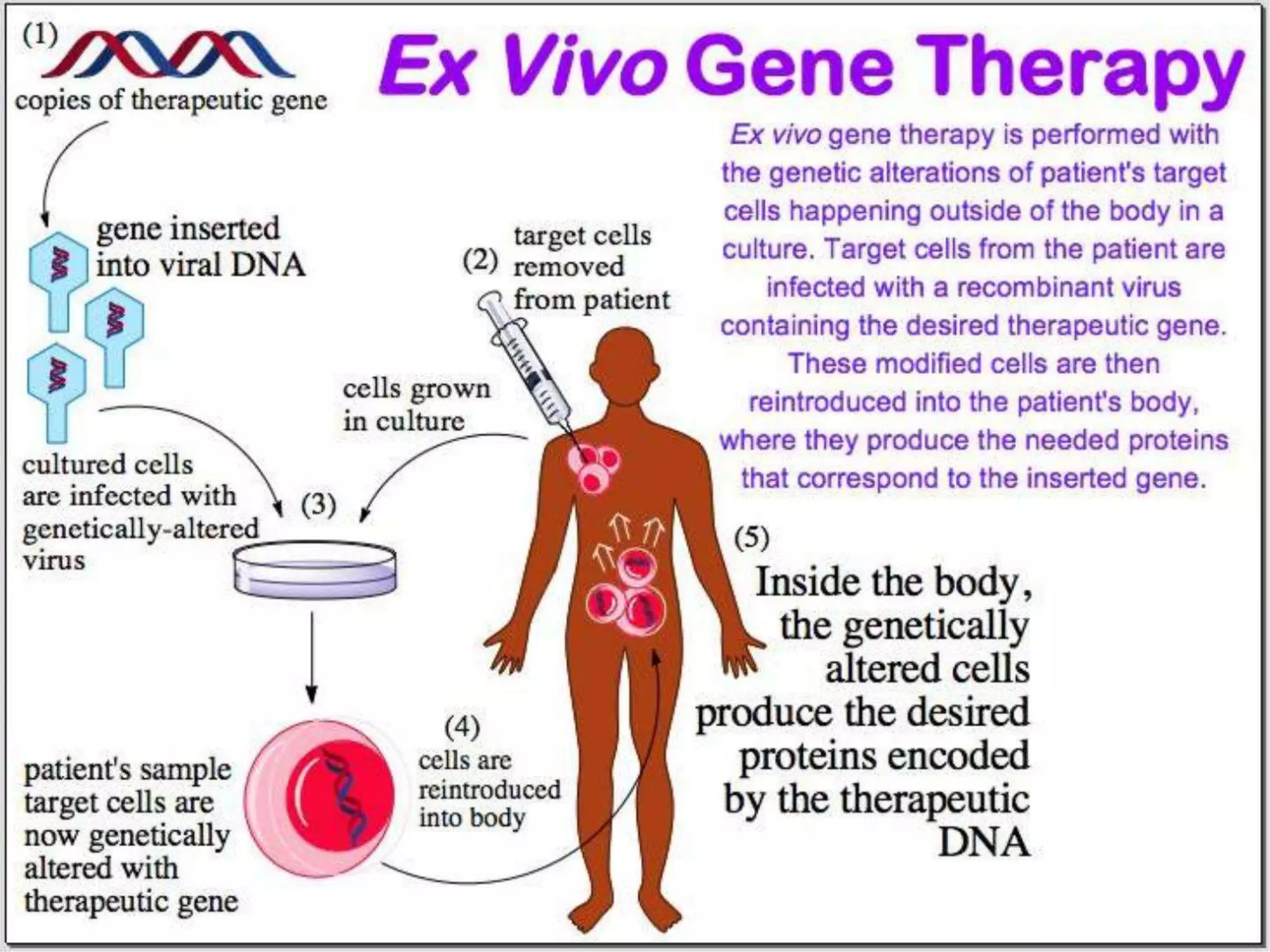
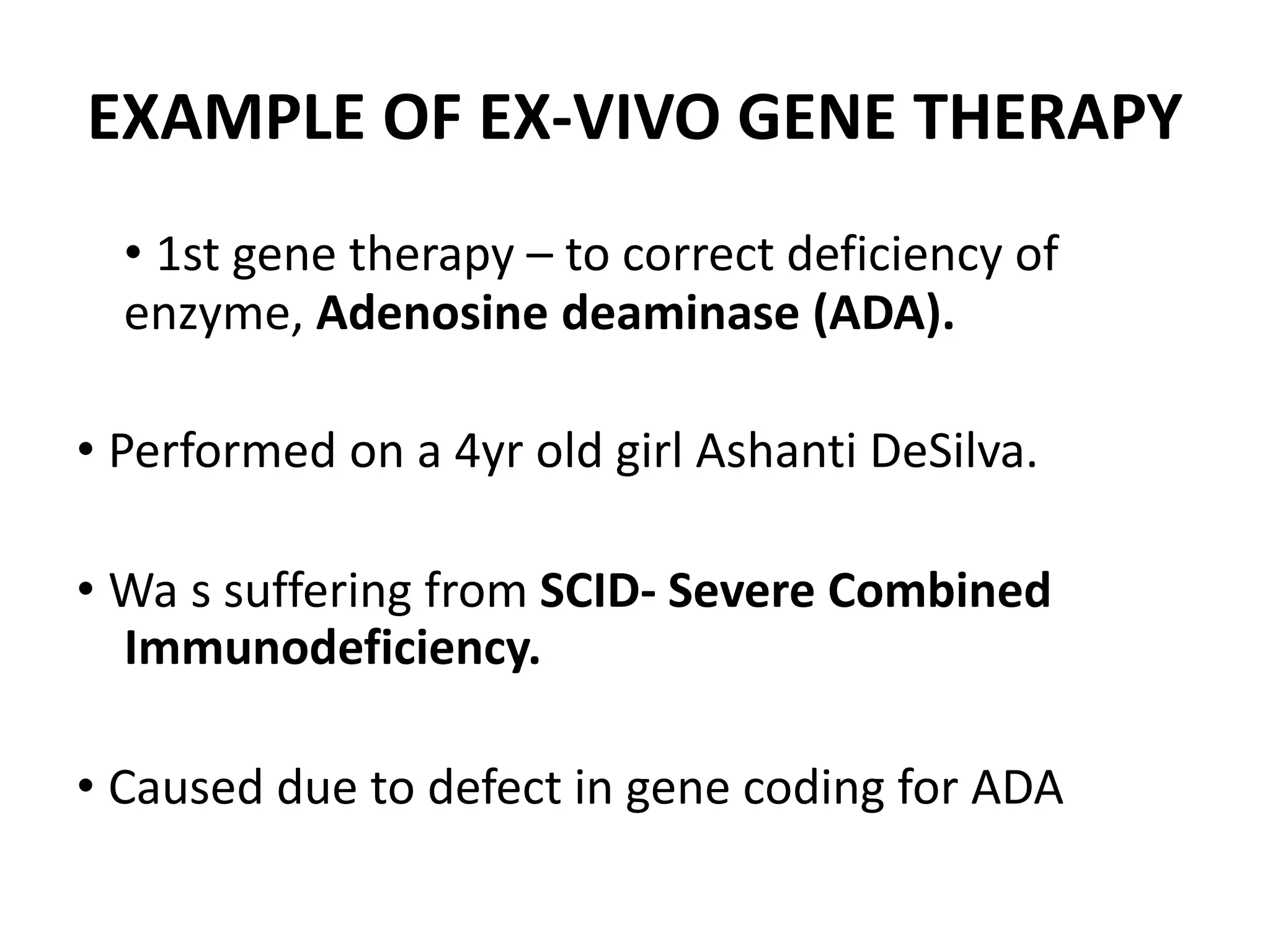
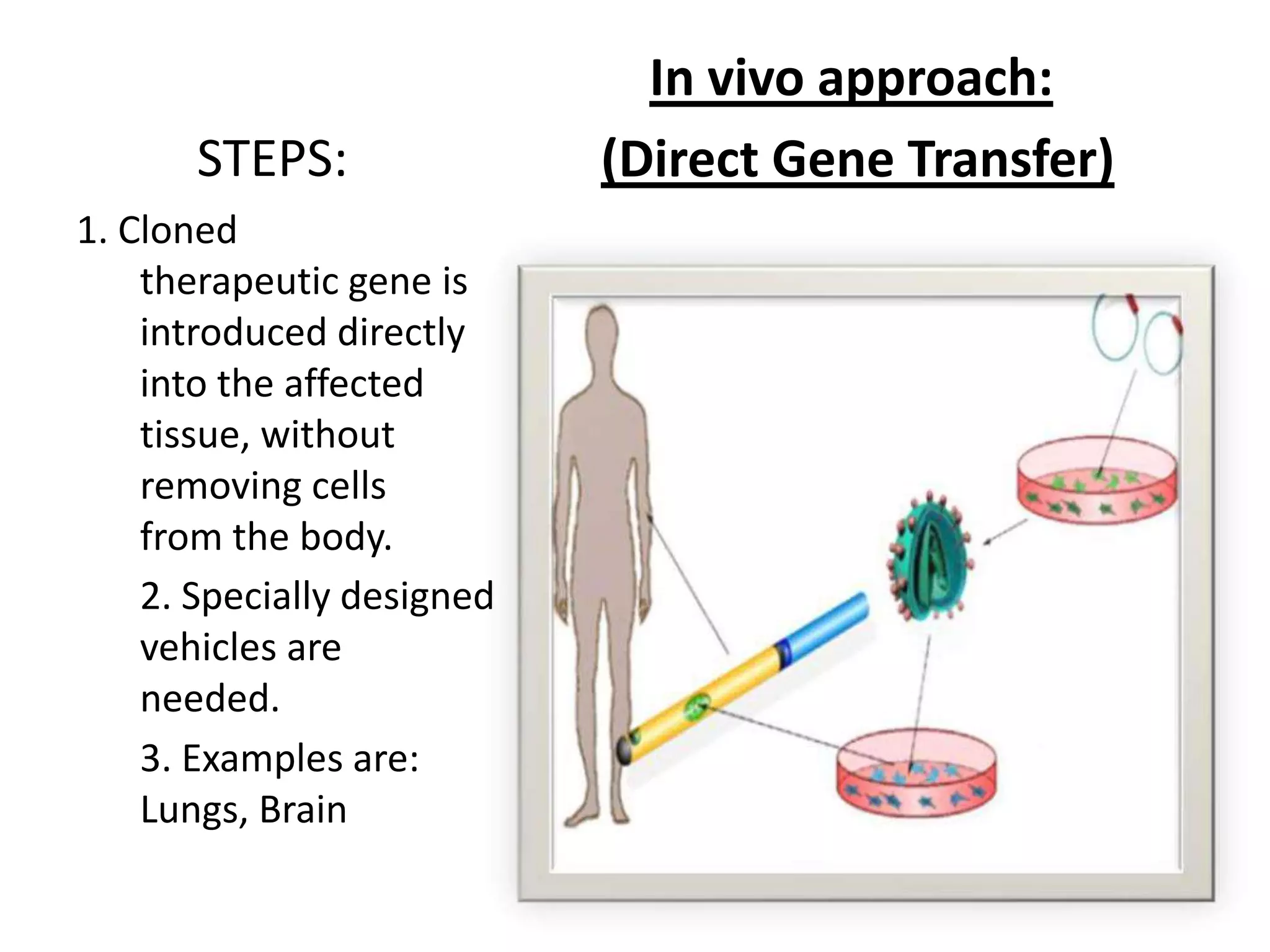
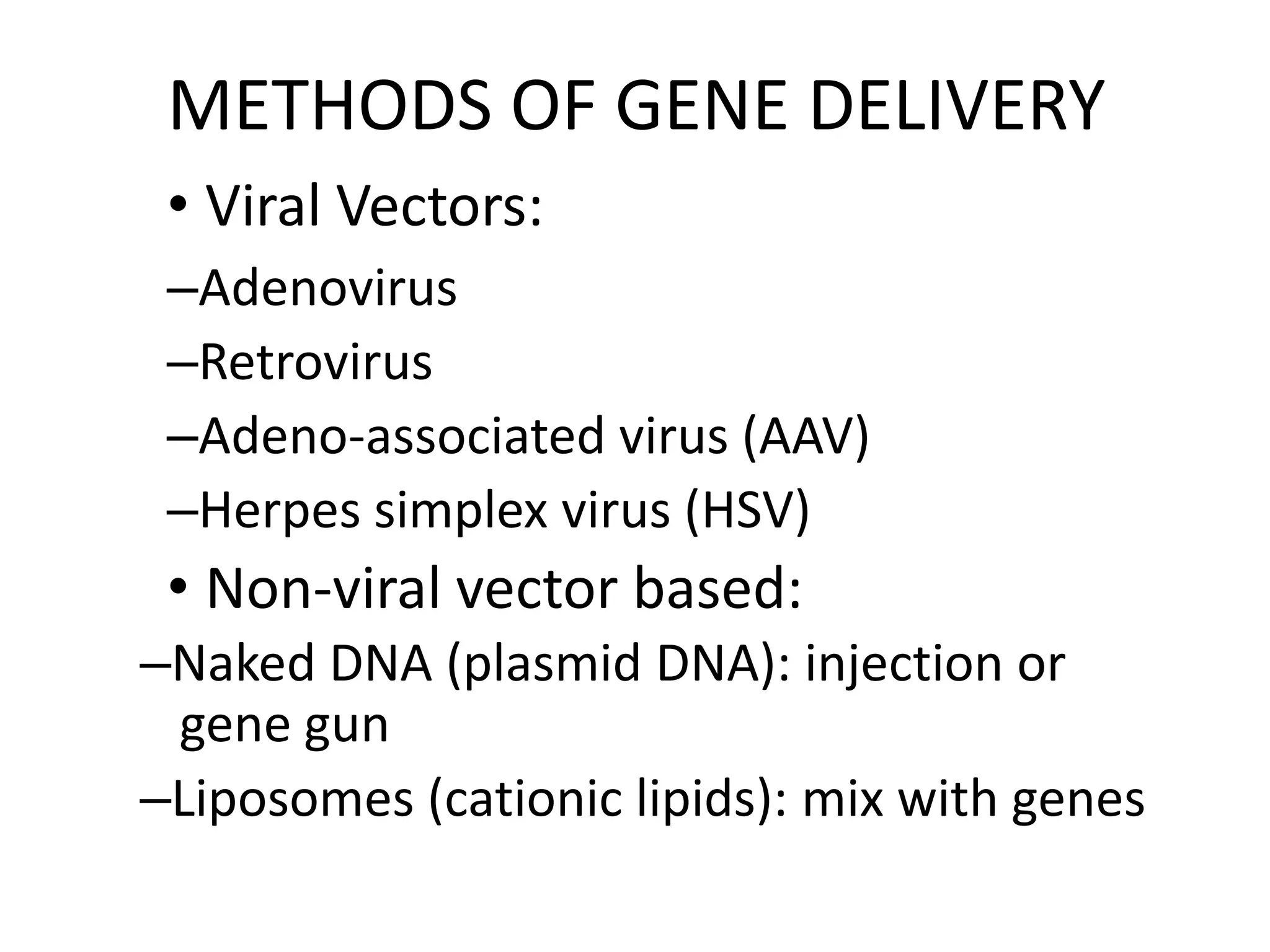
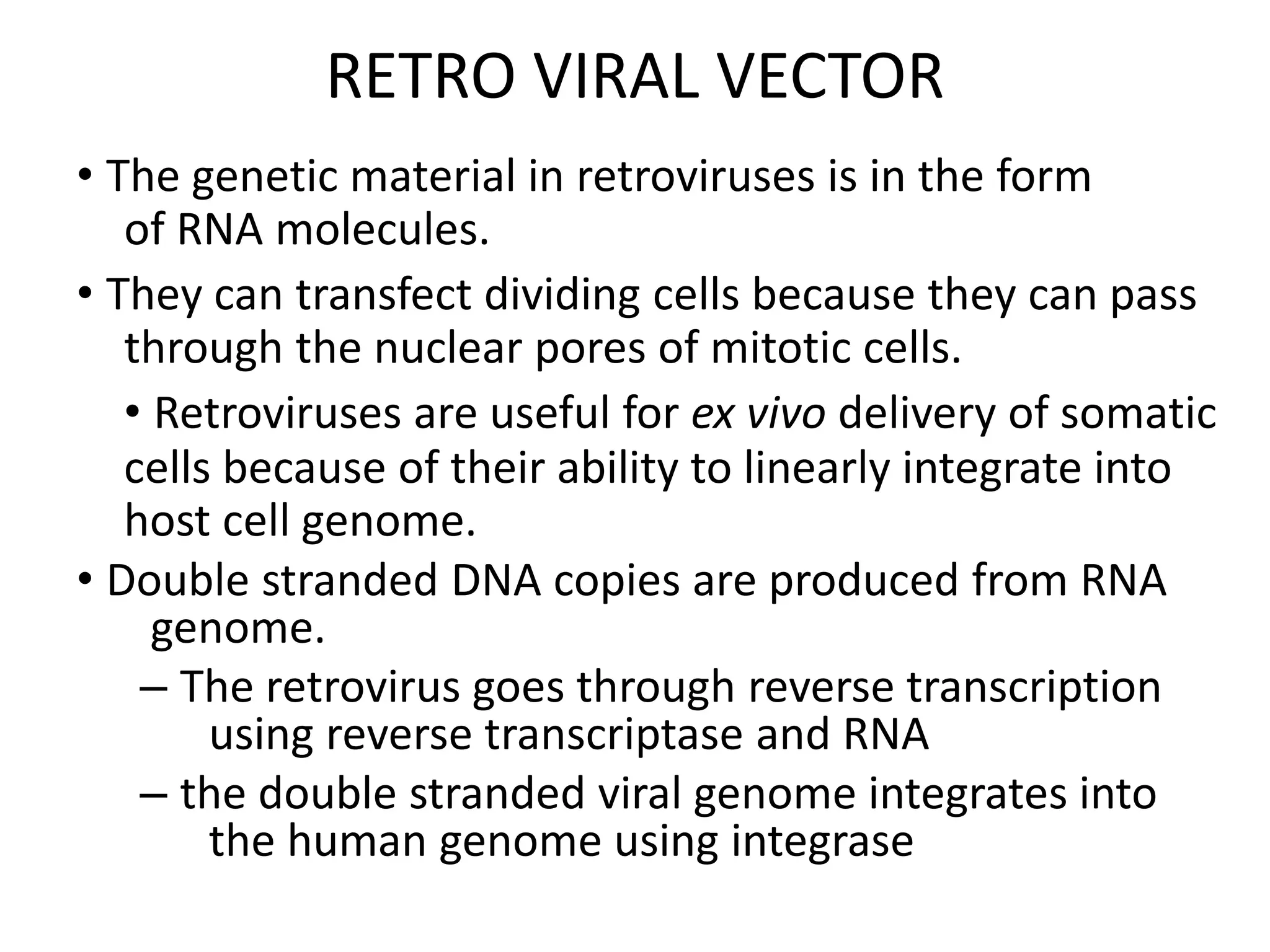
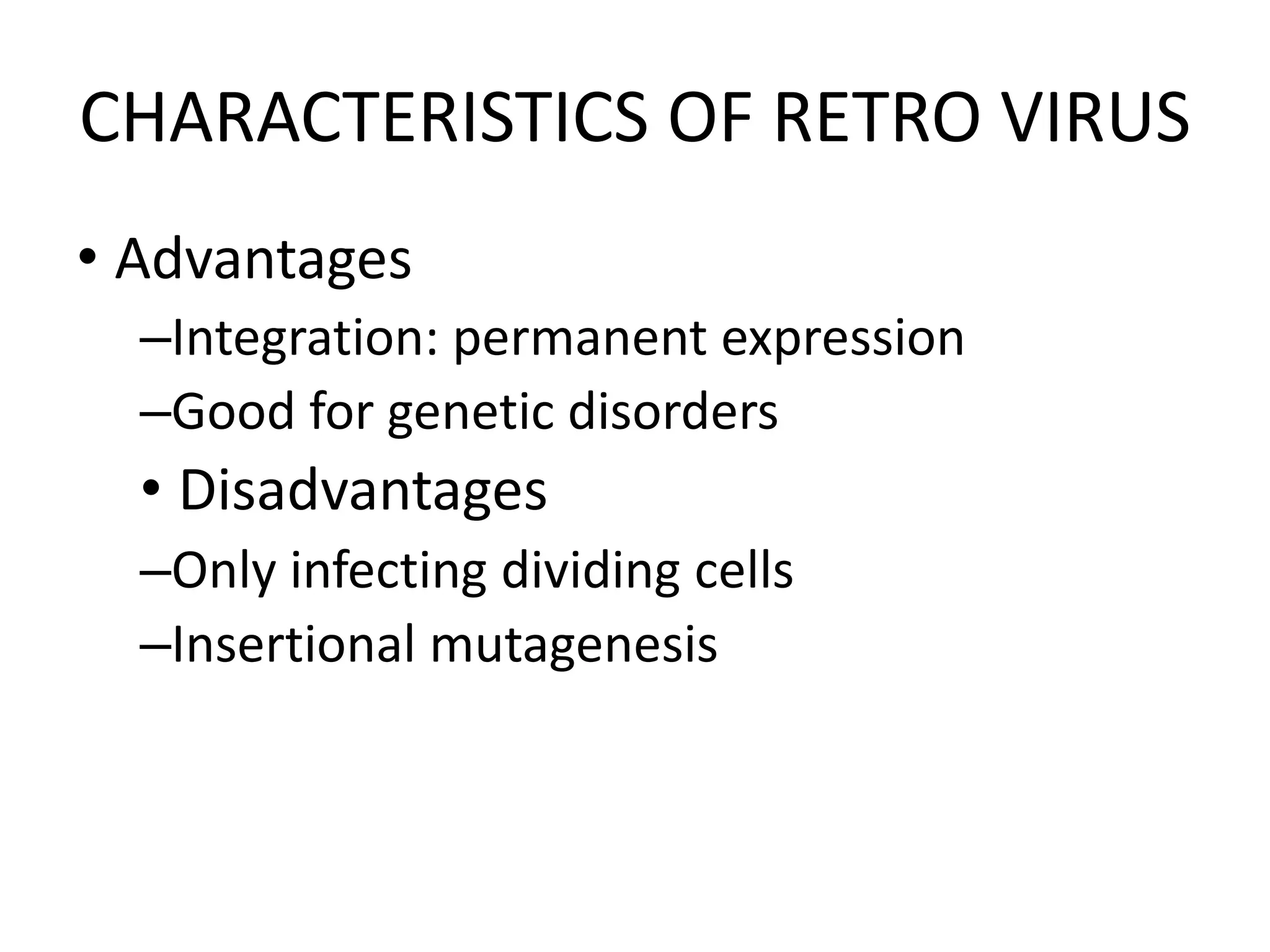
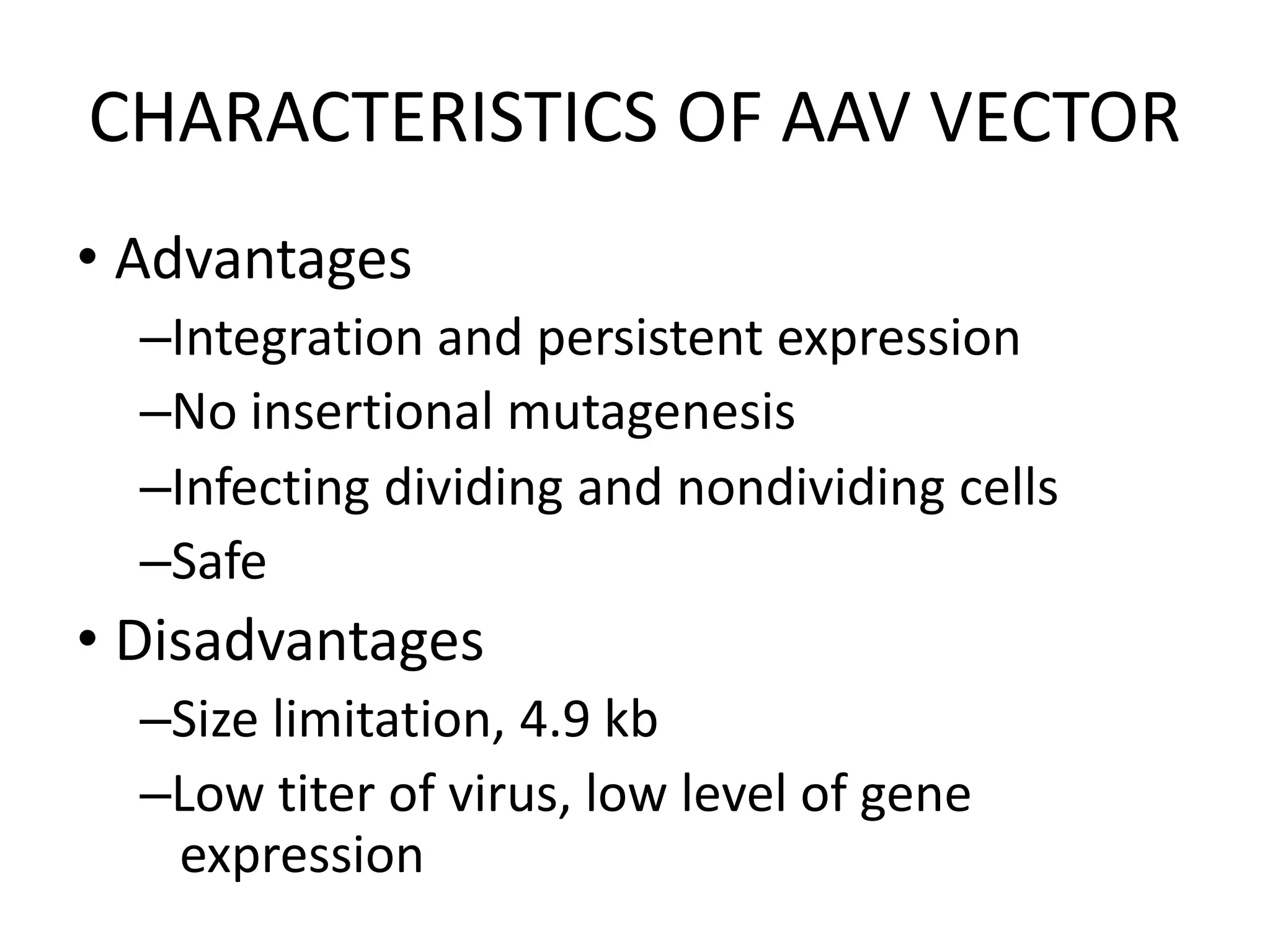
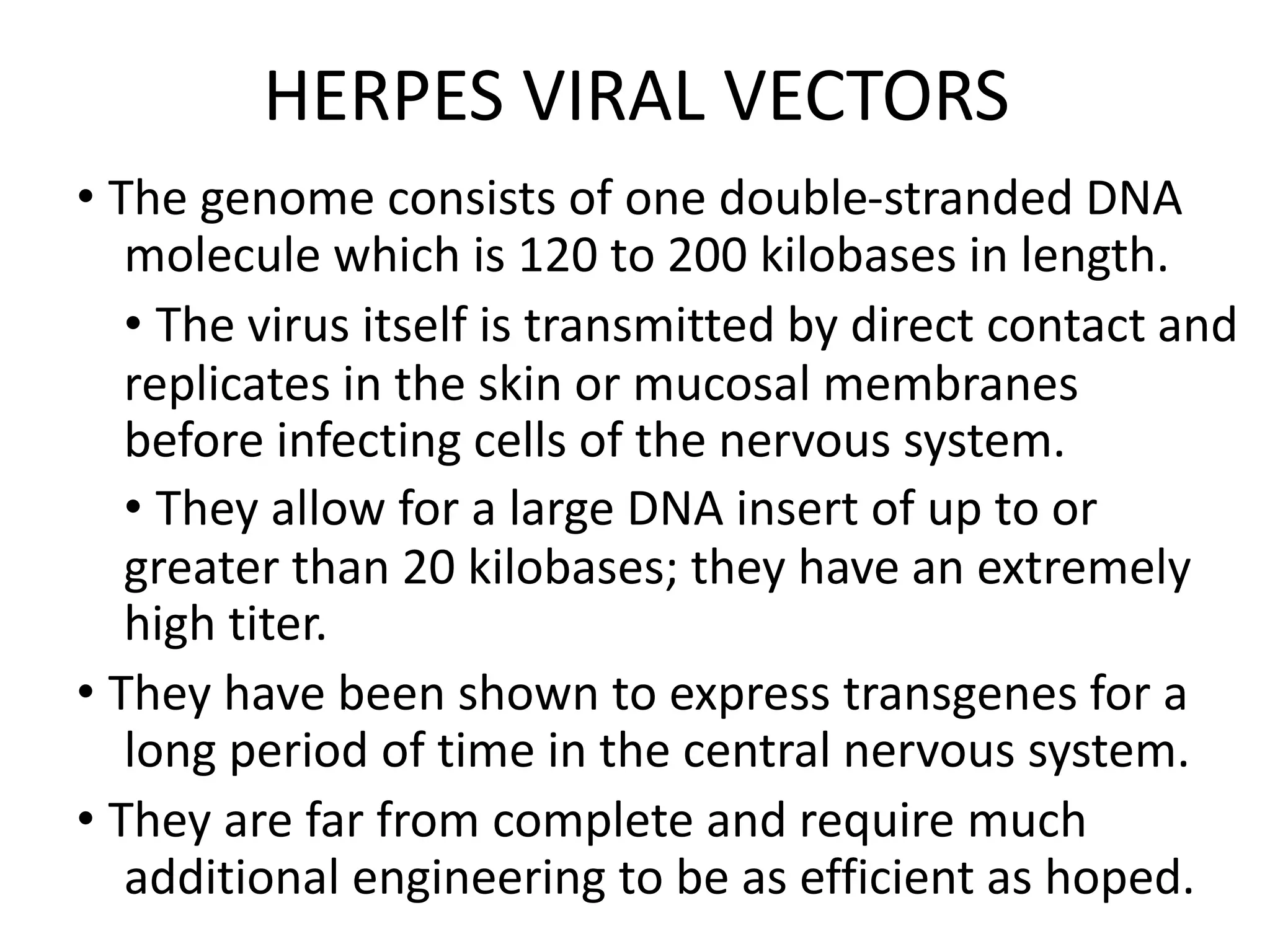
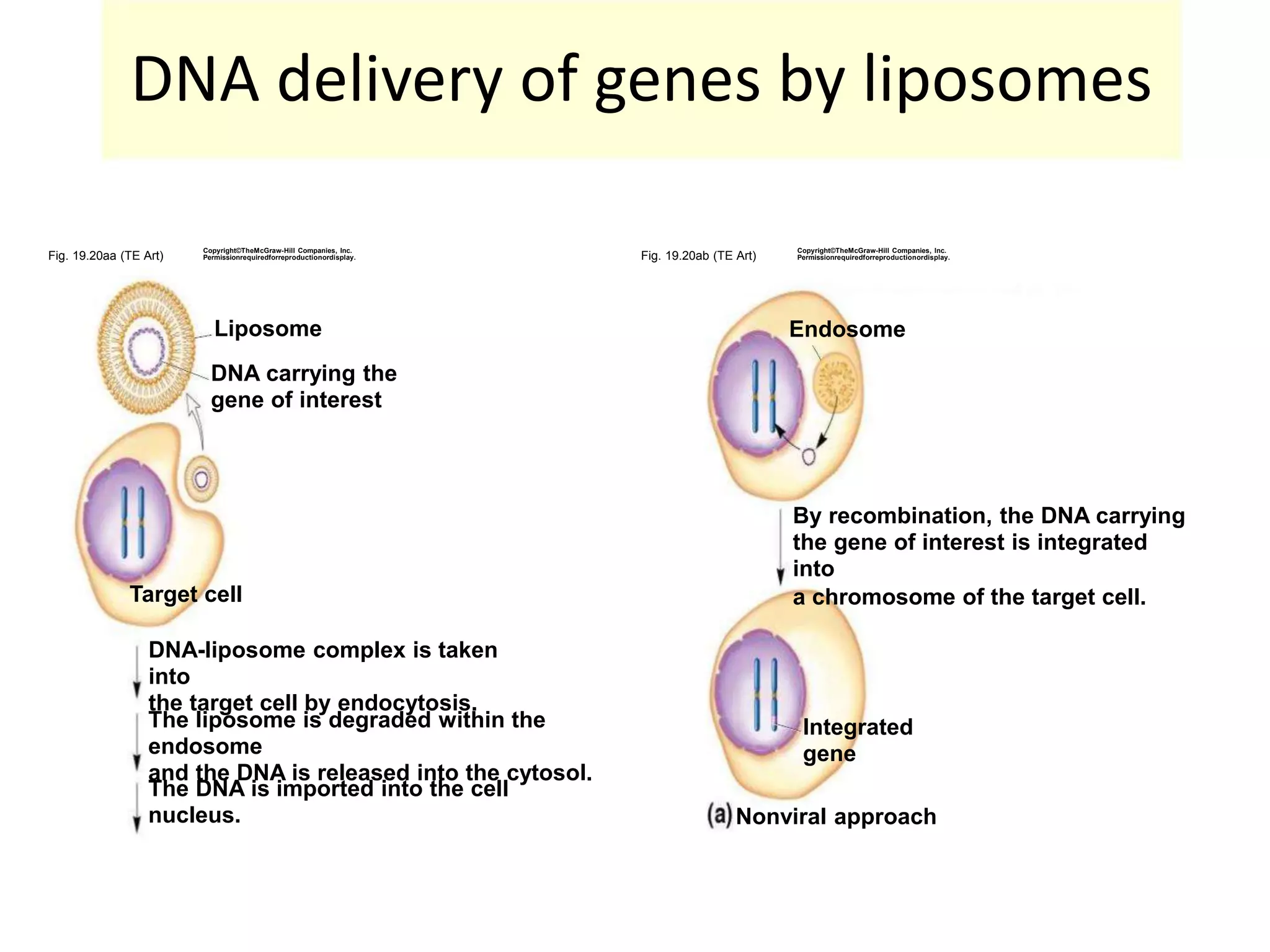
3. Gene therapy aims to treat diseases caused by defective genes by inserting functional genes. It can target somatic or germ-line cells. Viral and non-viral vectors are used to deliver therapeutic genes.