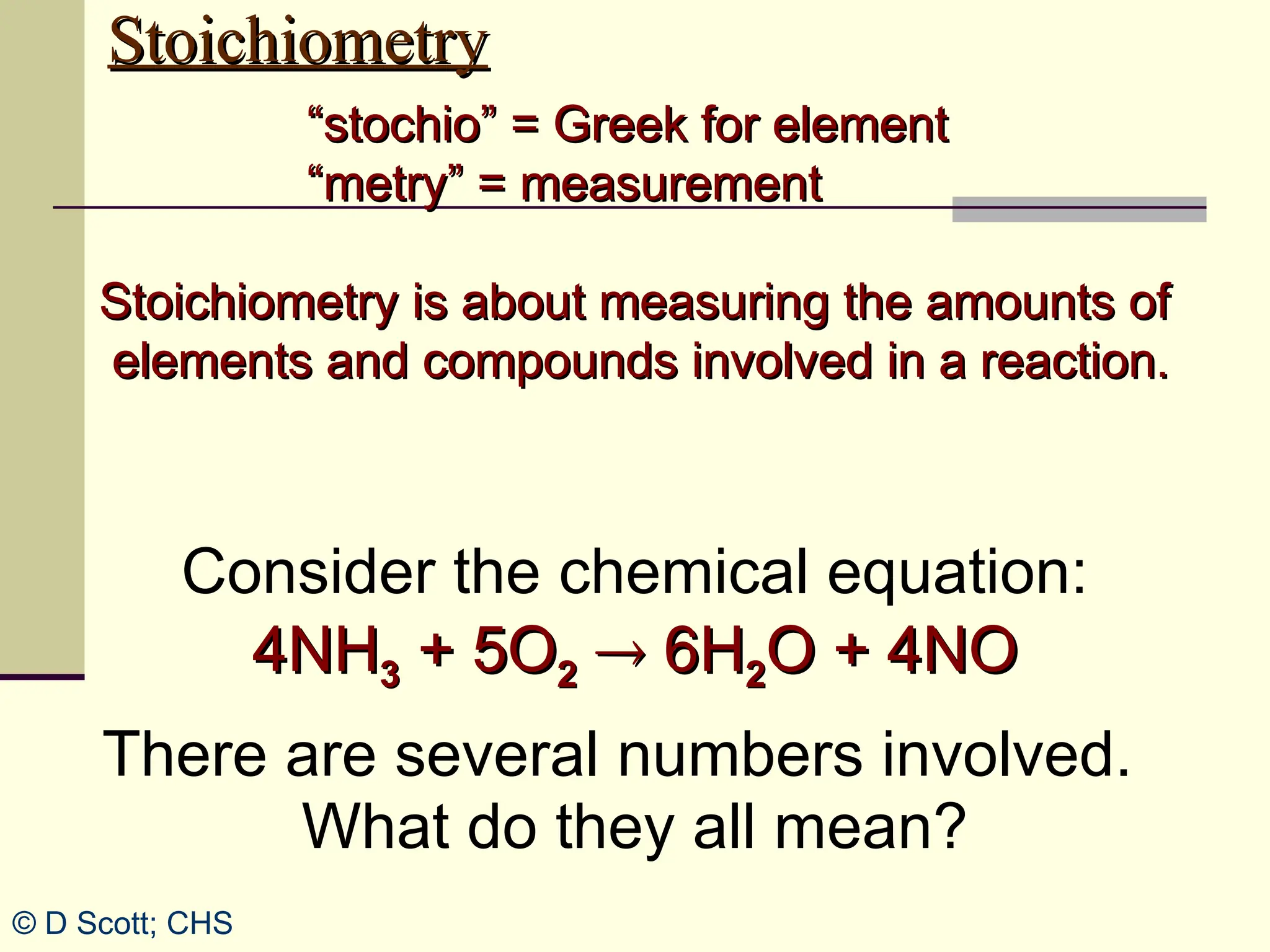
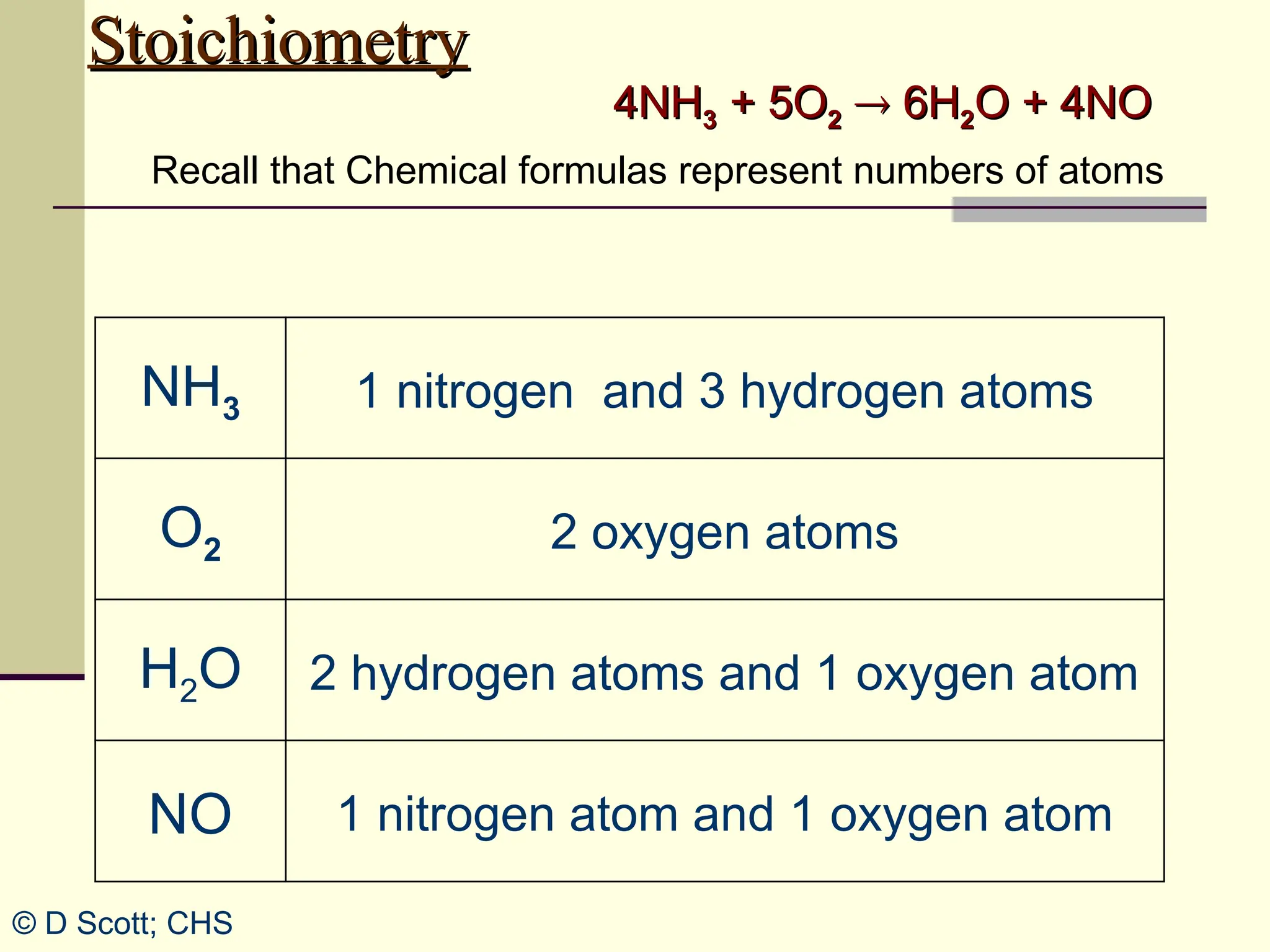
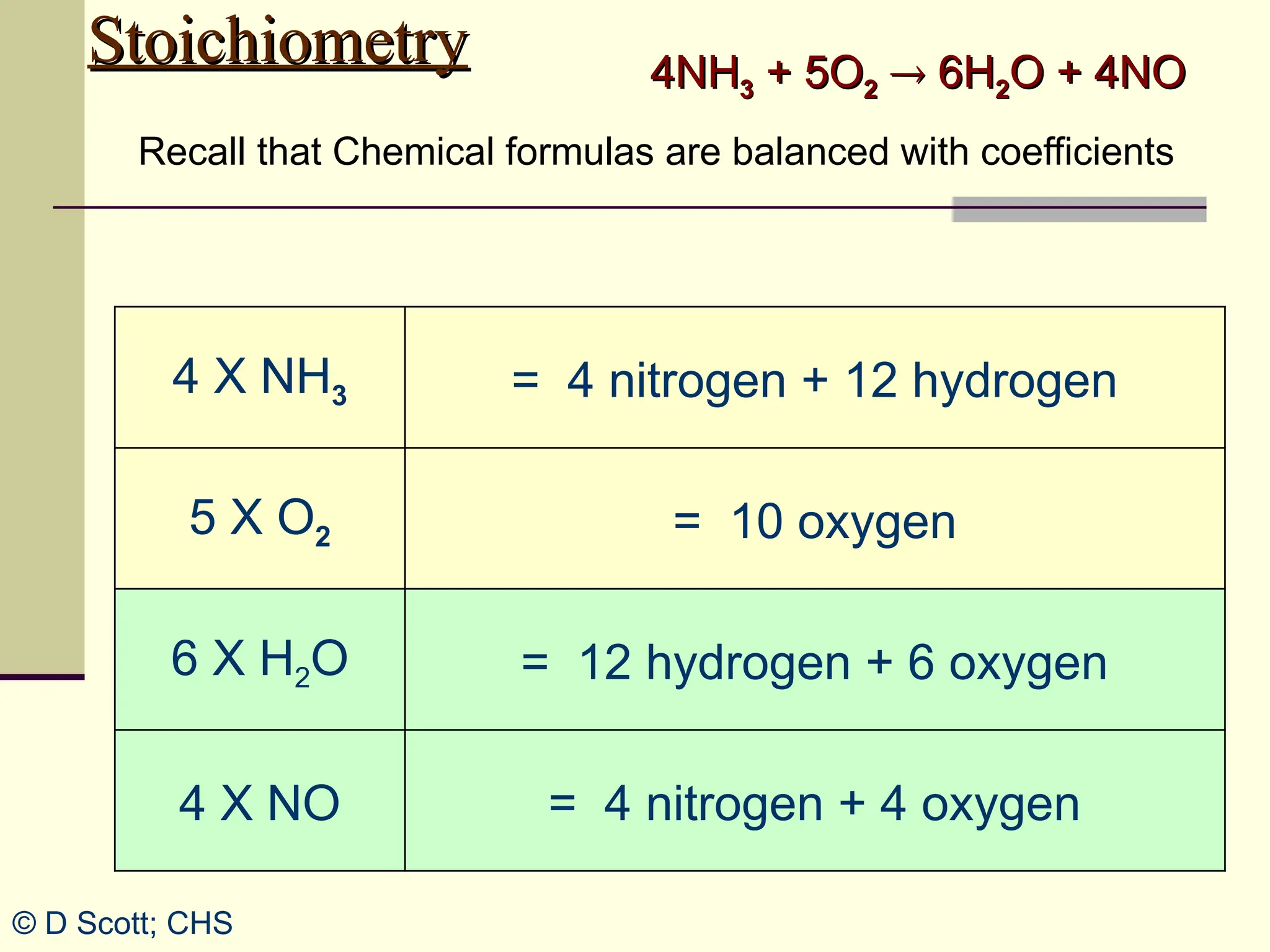
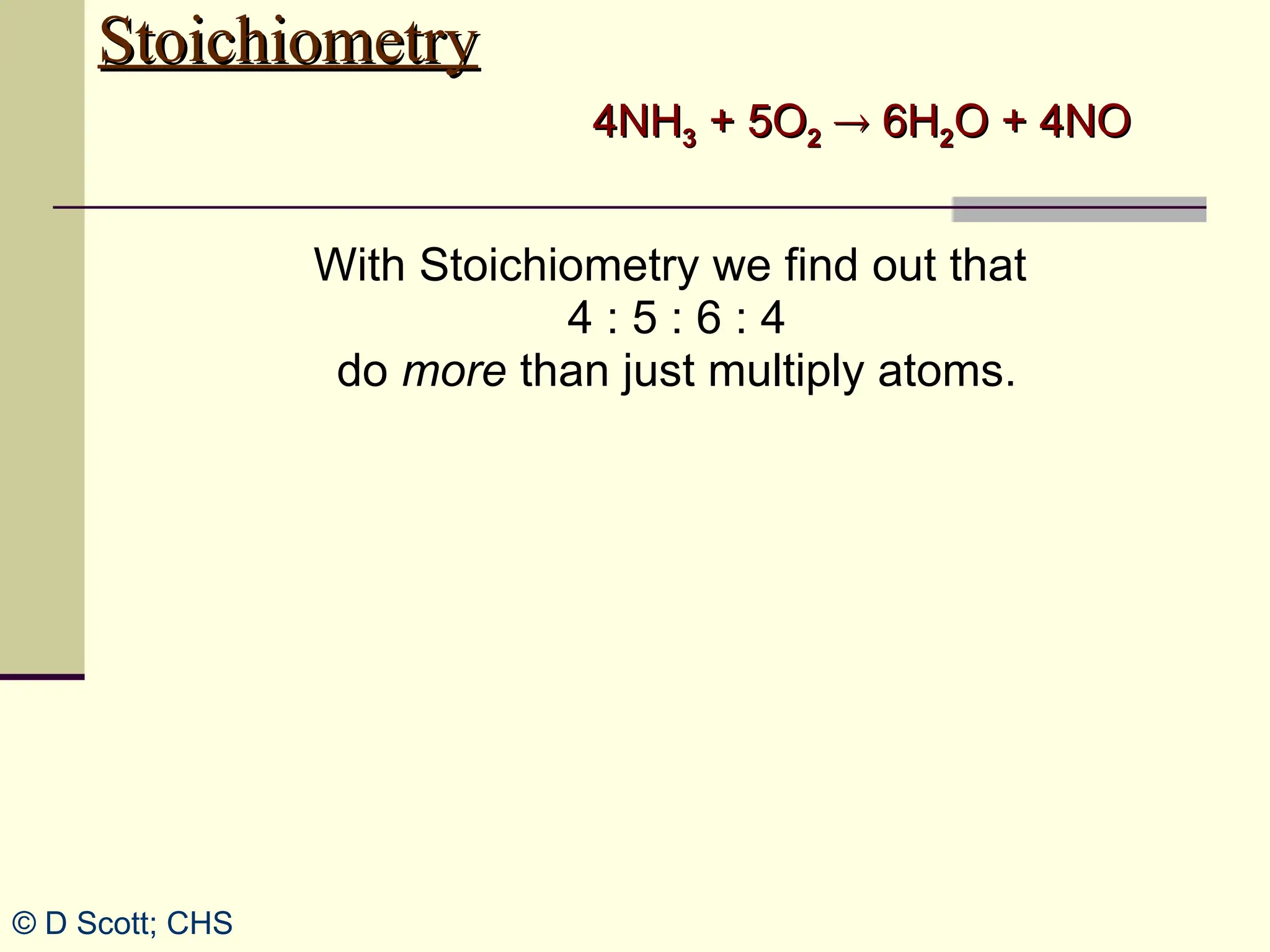
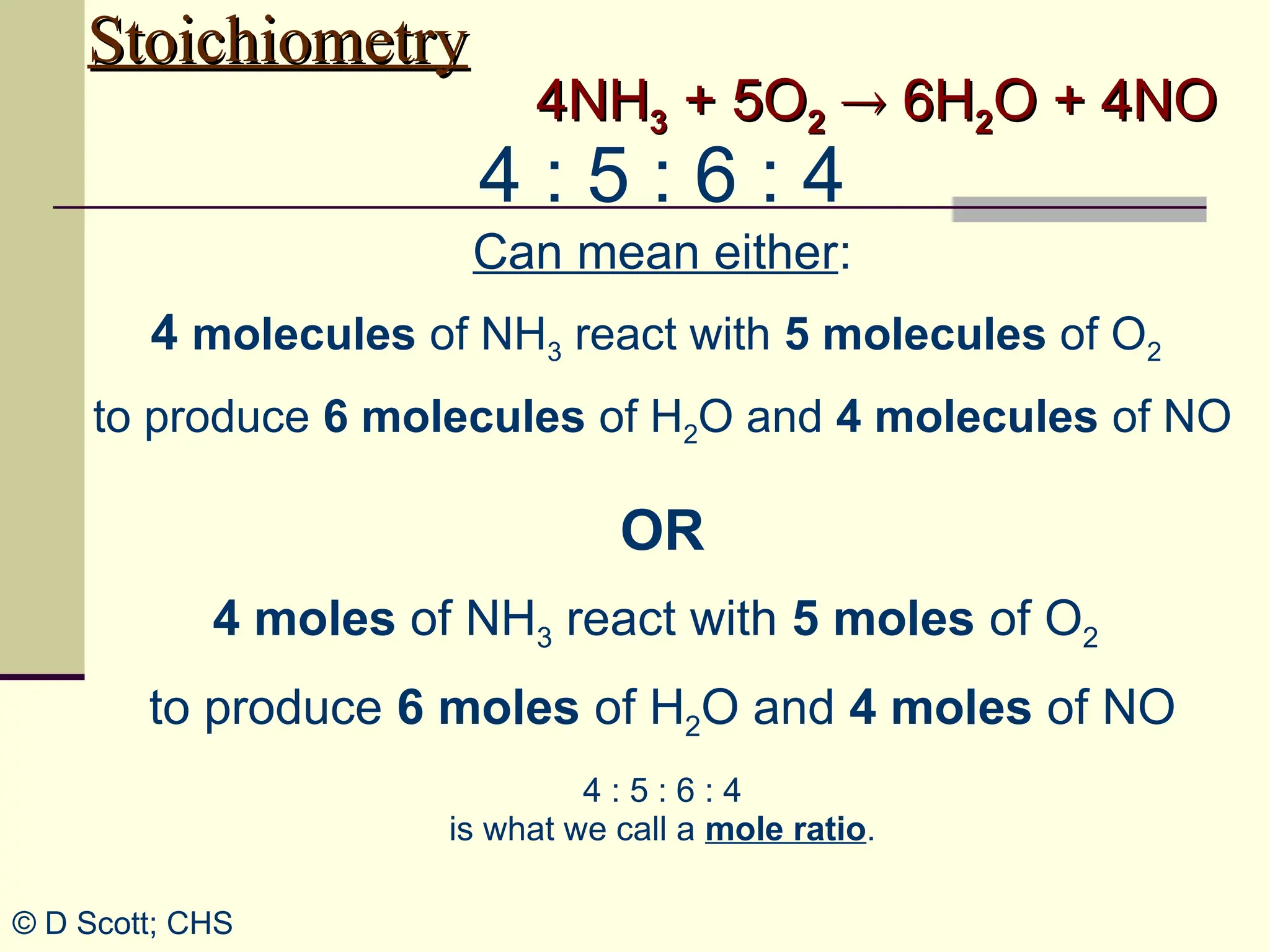
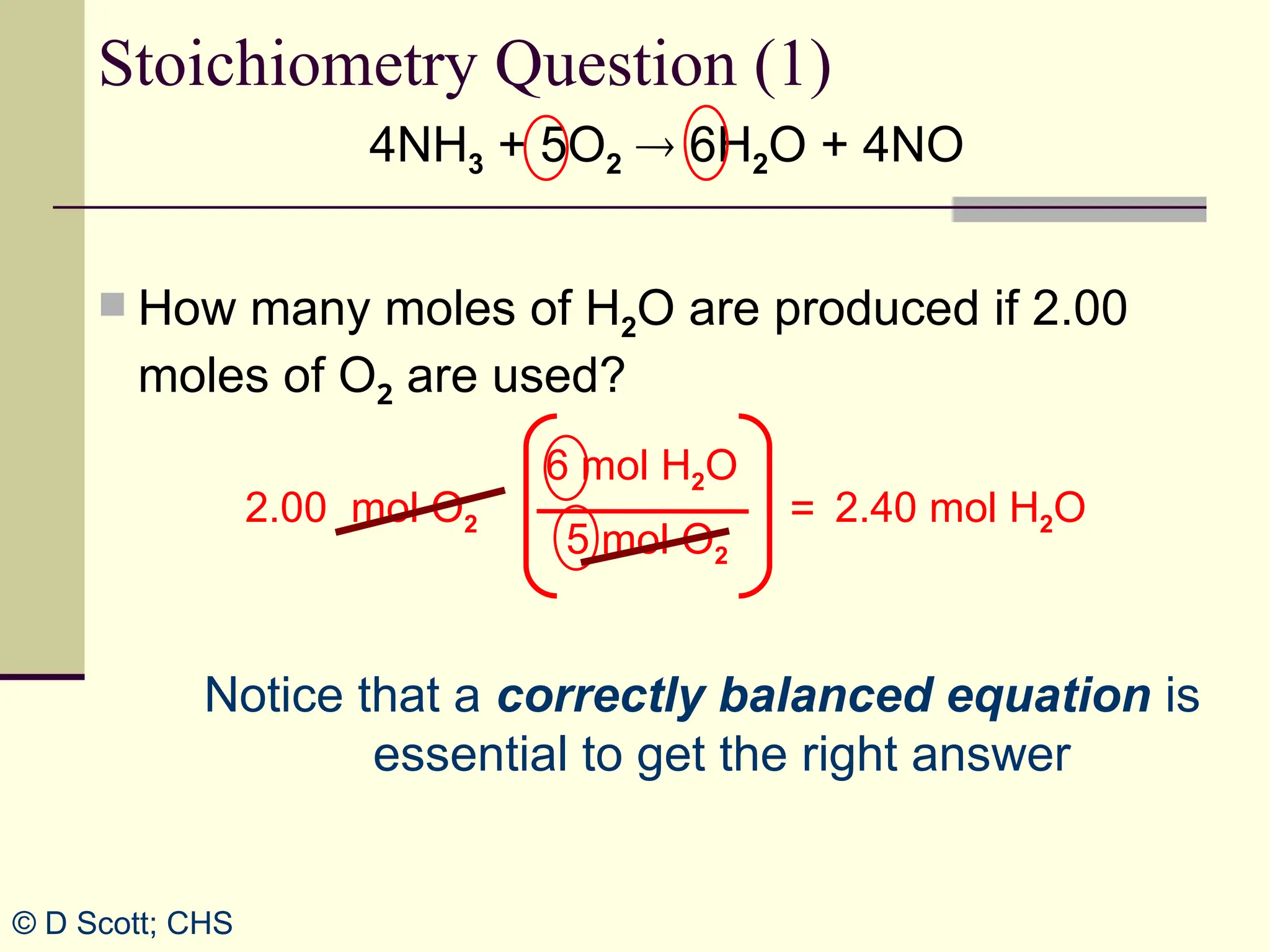
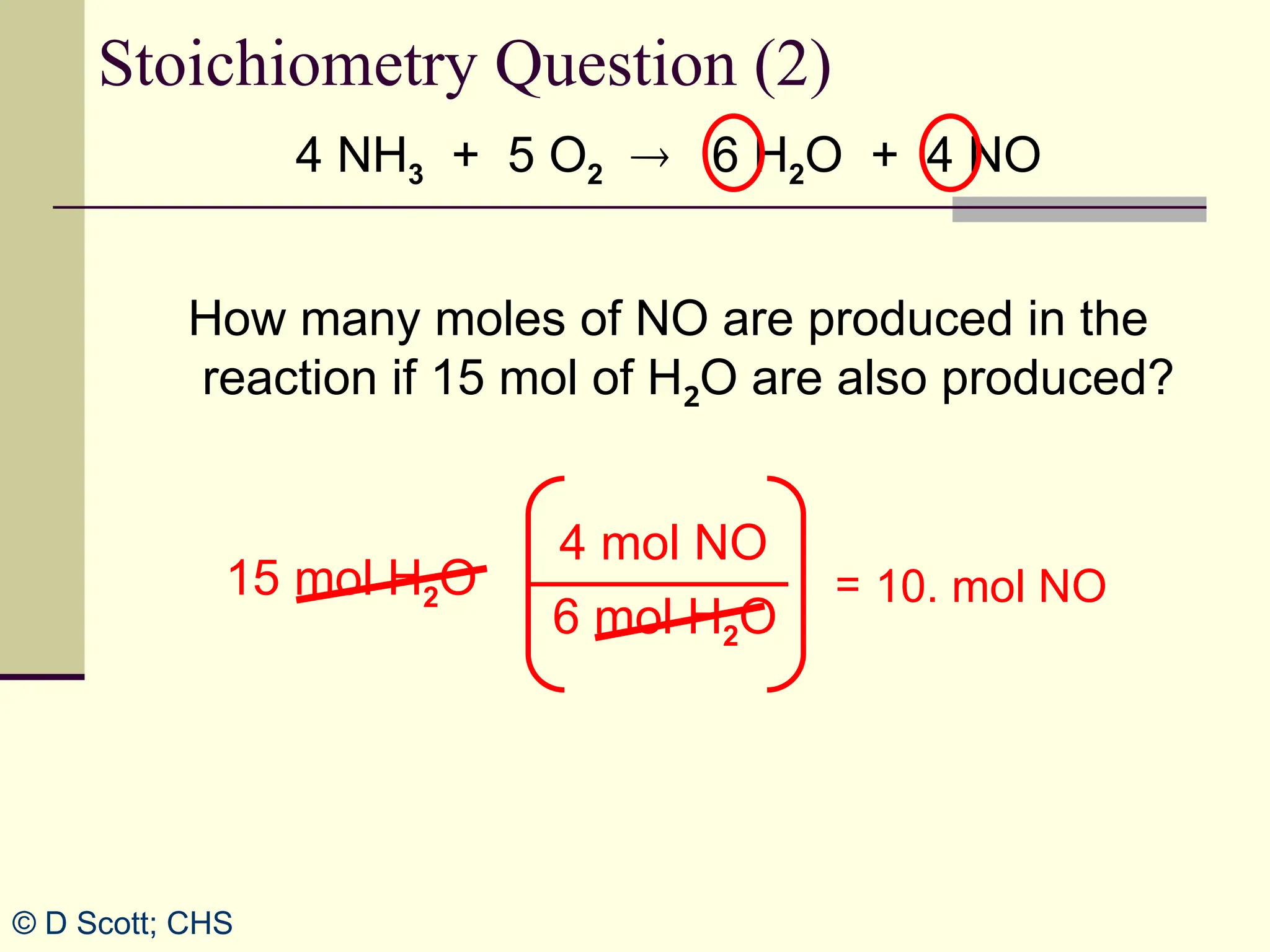
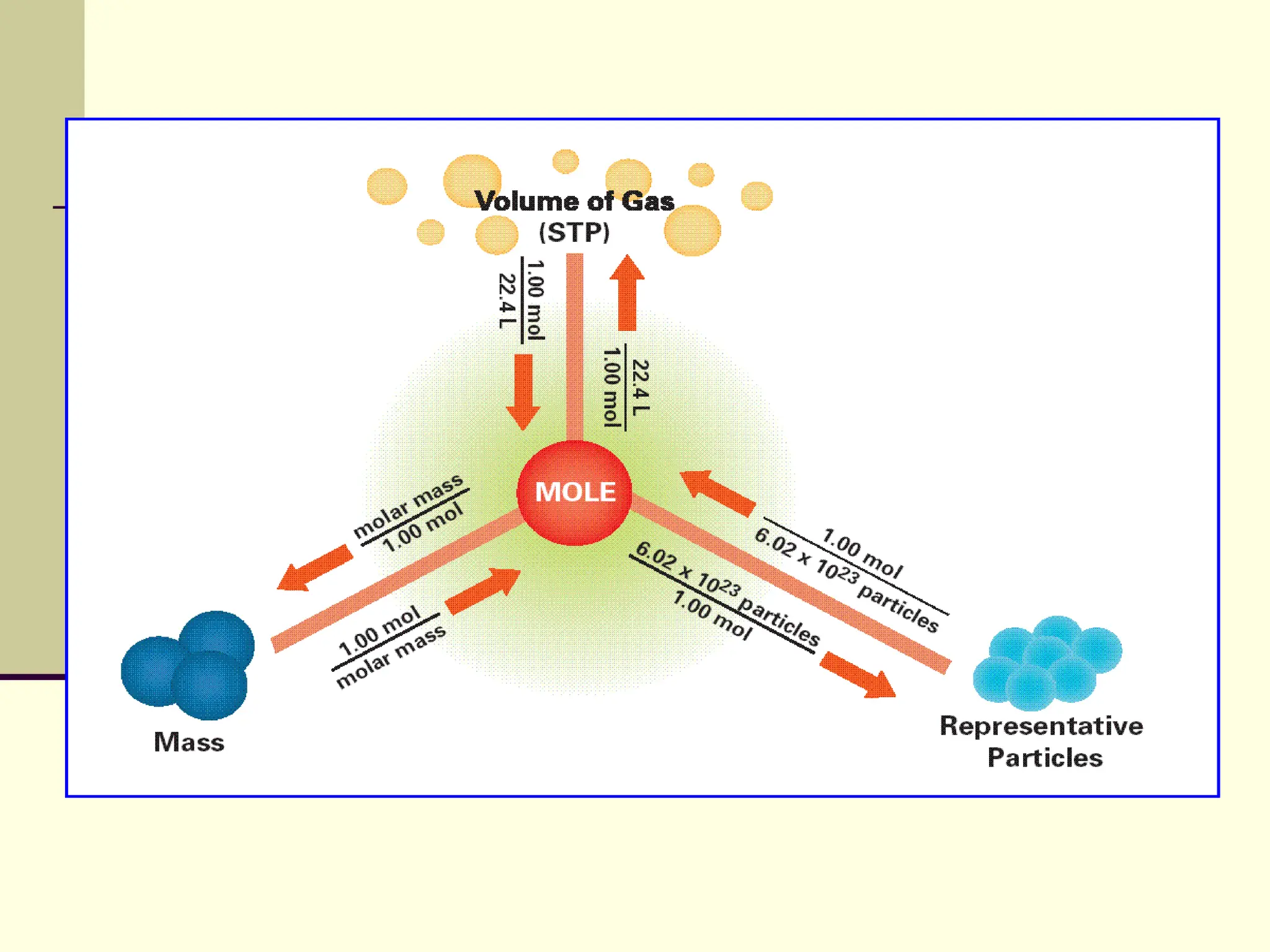
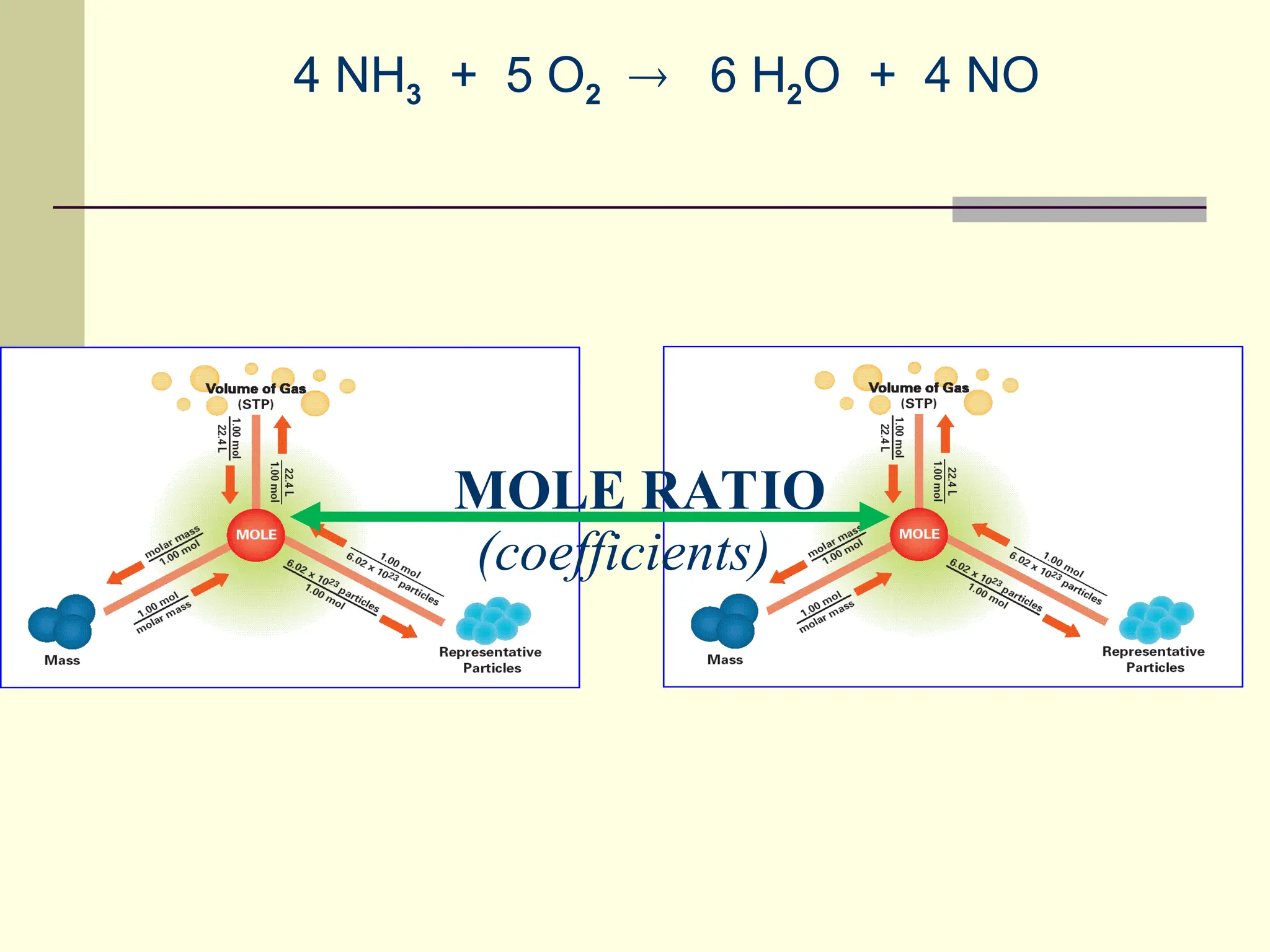
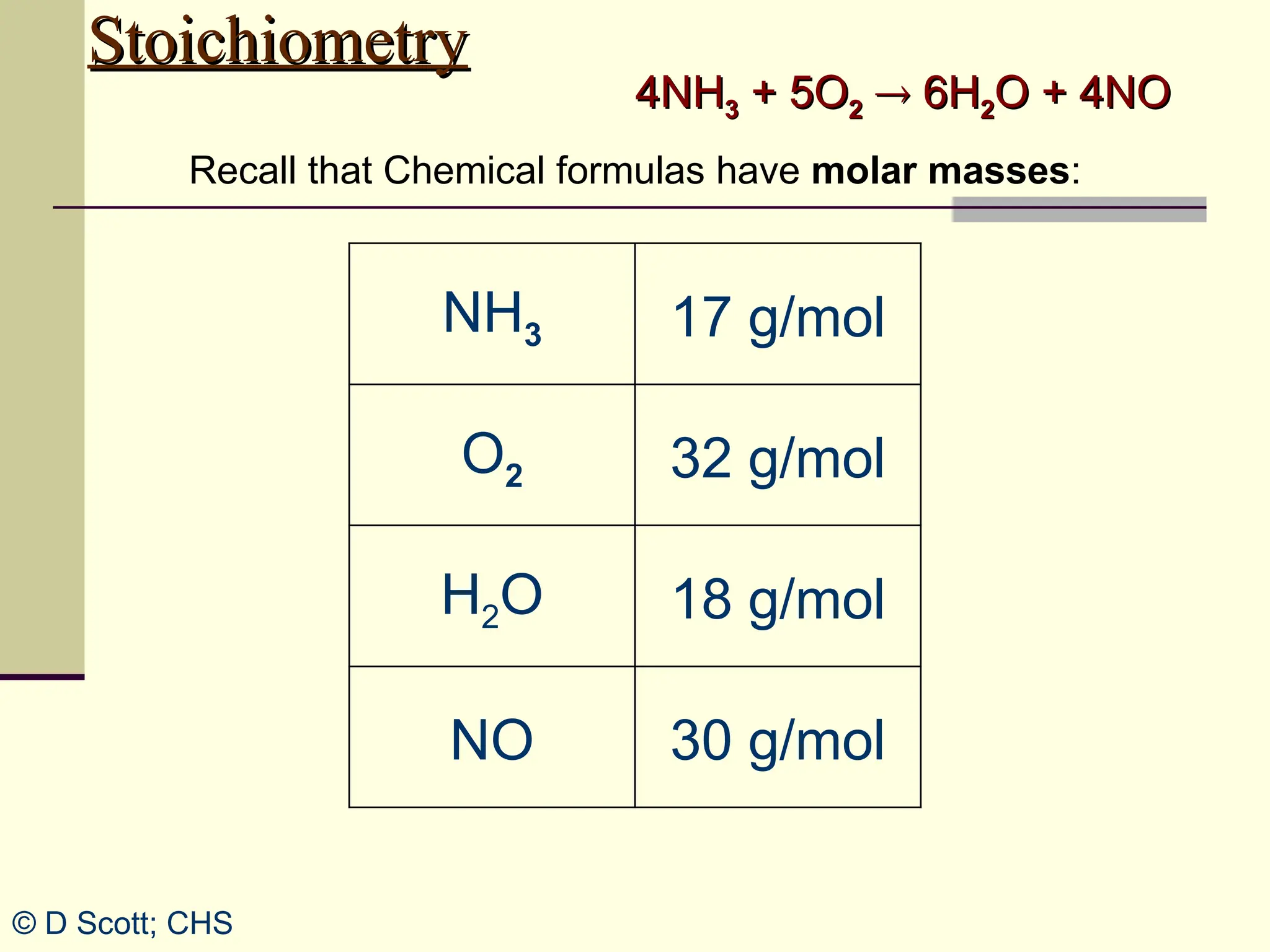
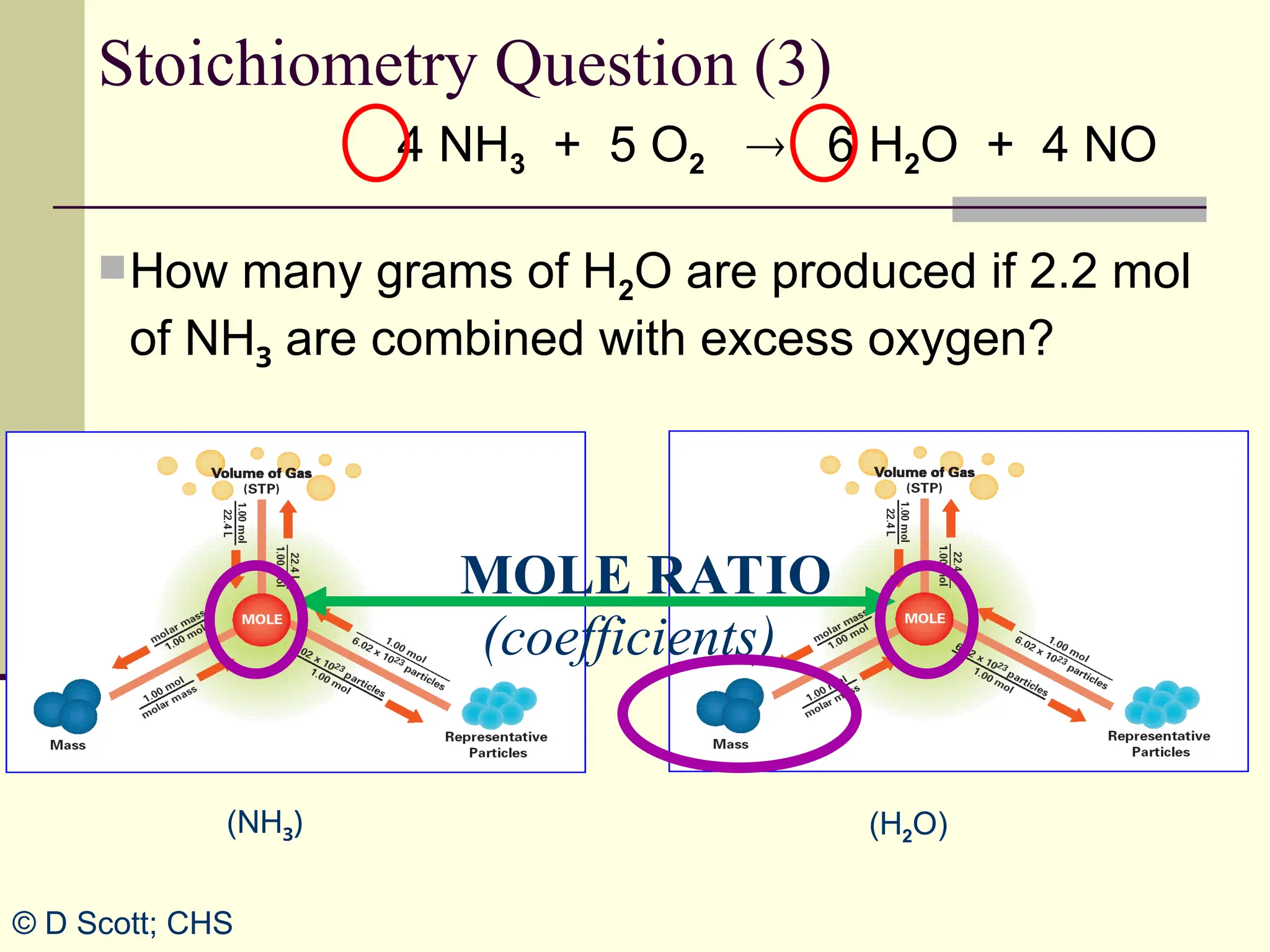
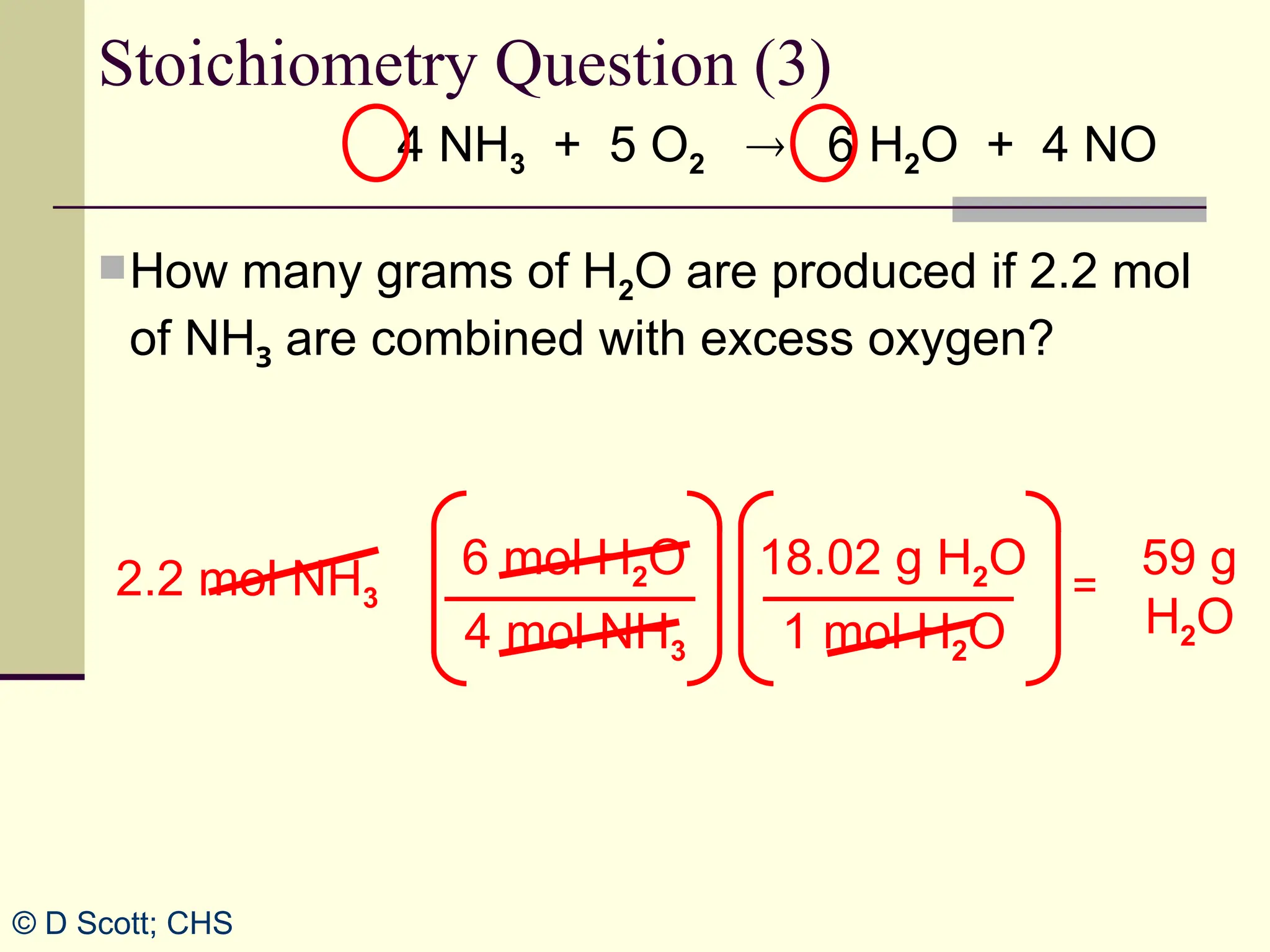
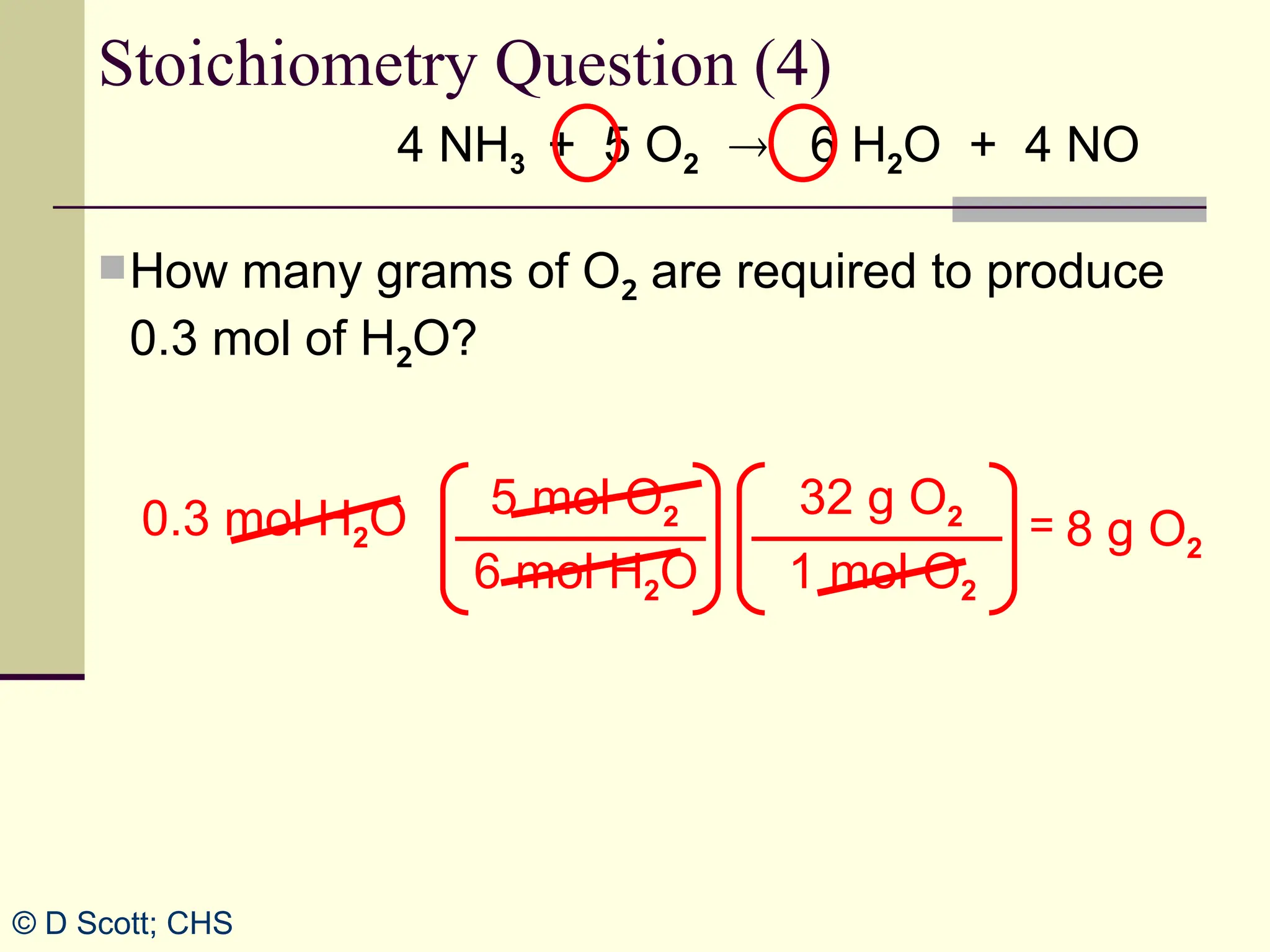
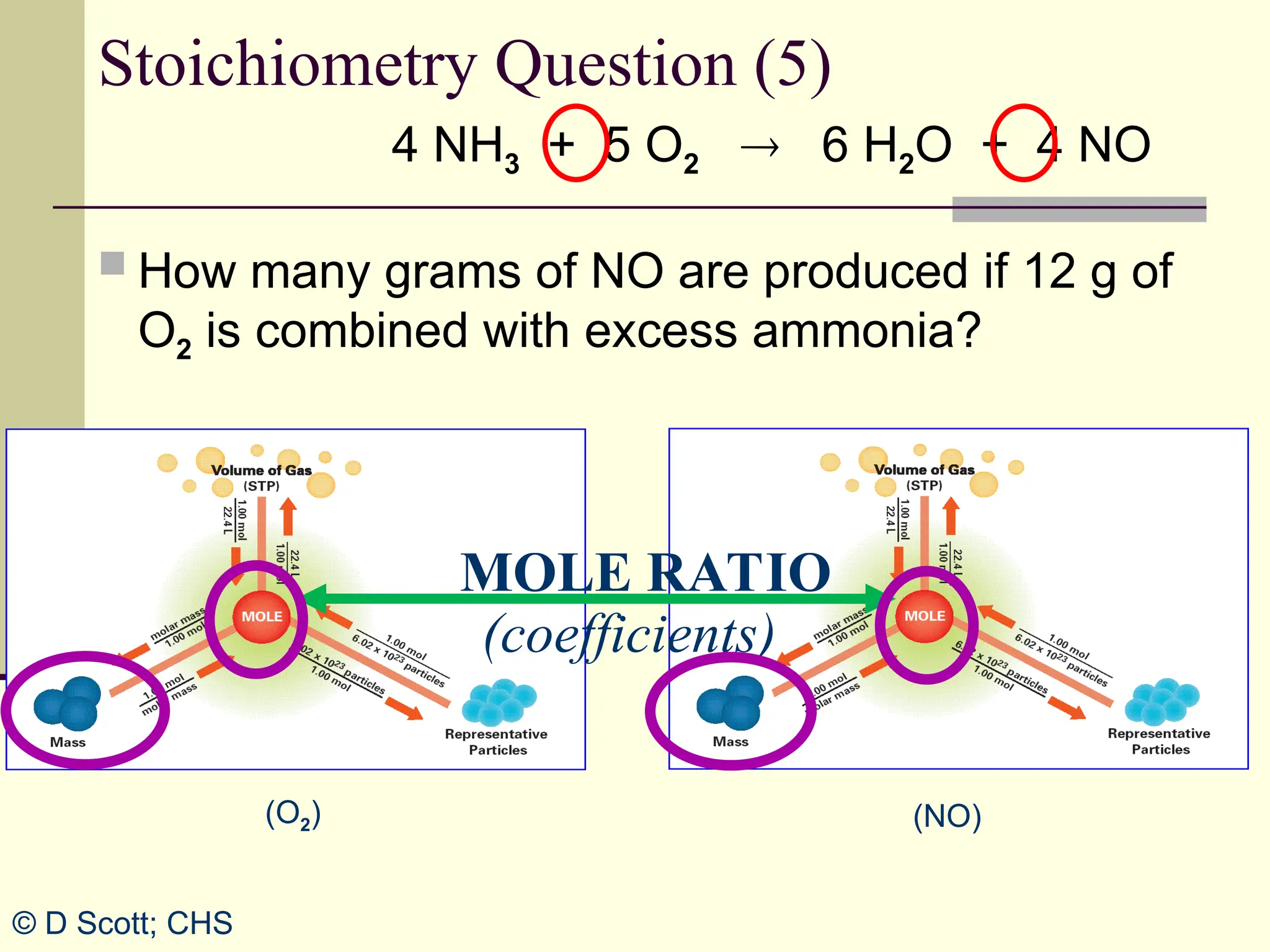
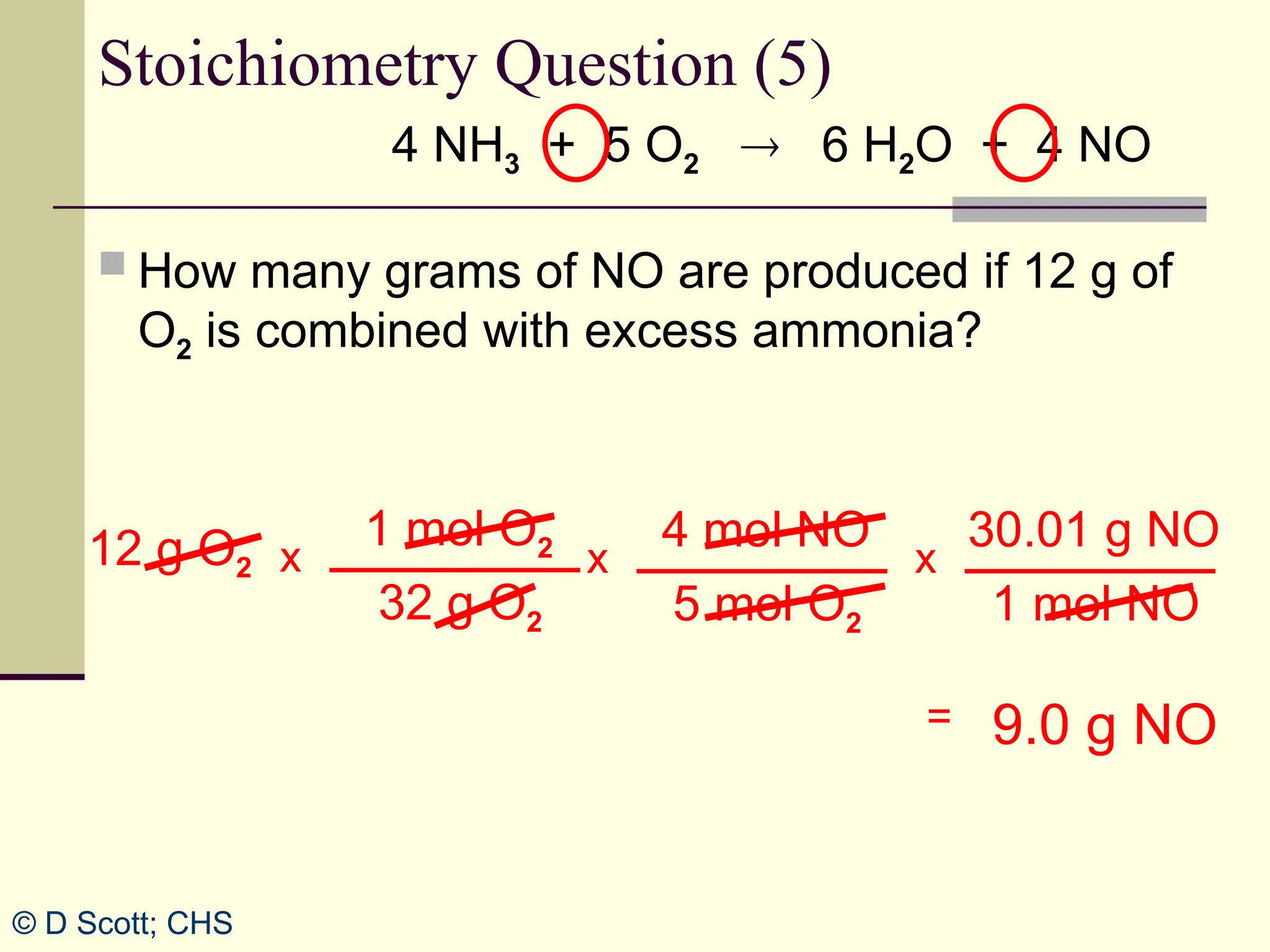
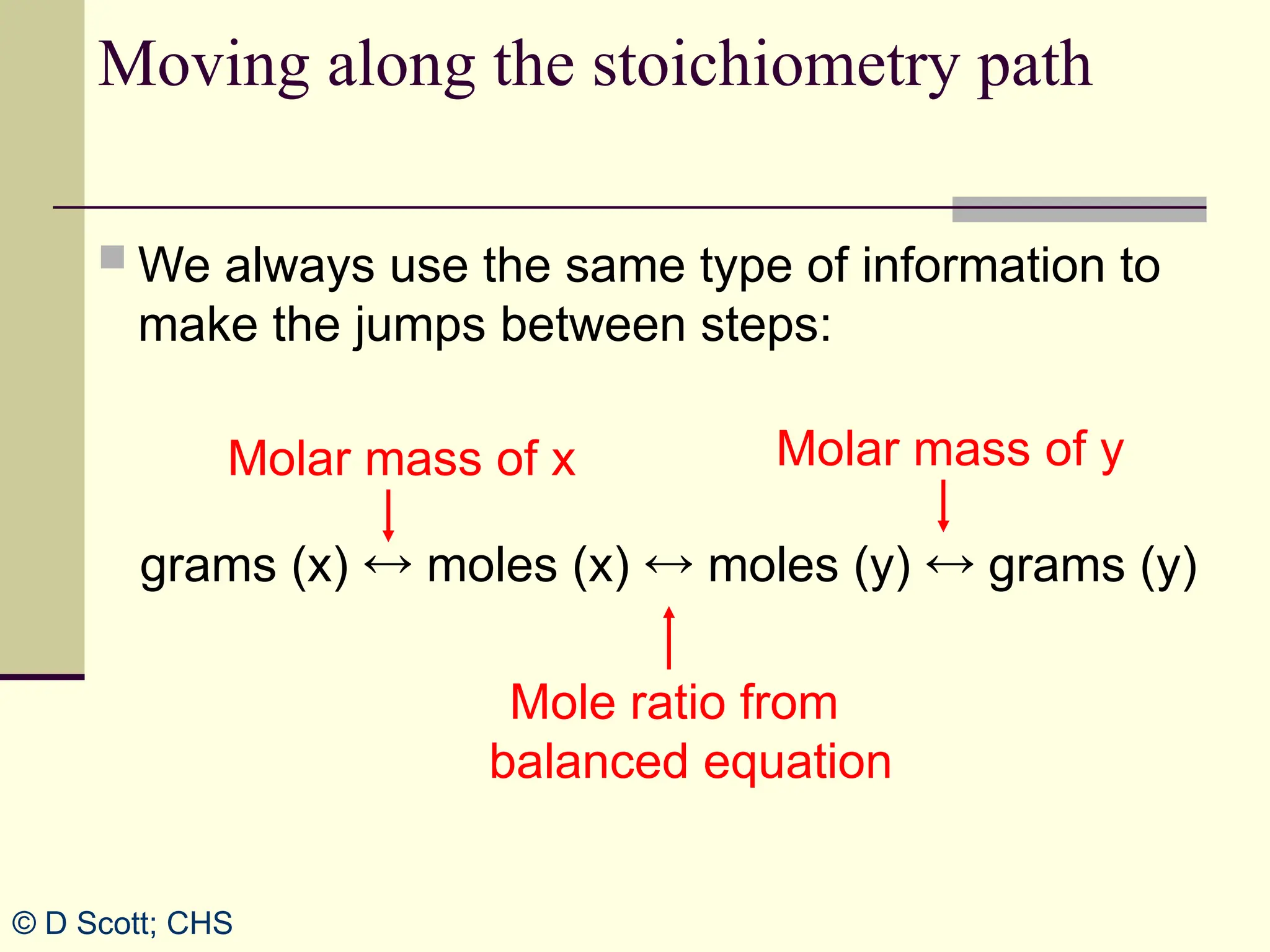
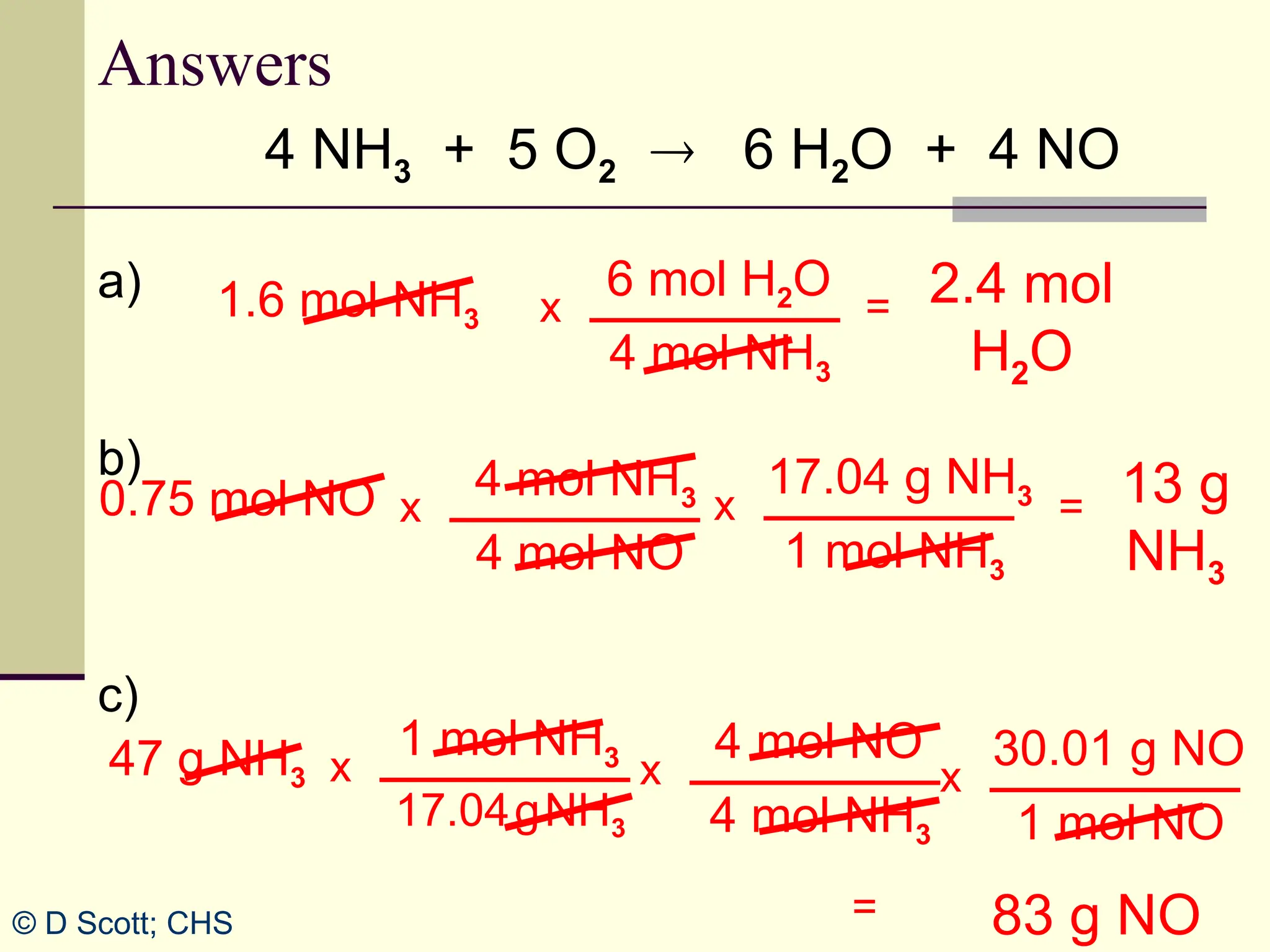
The document discusses stoichiometry, explaining it as the measurement of the amounts of elements and compounds involved in a chemical reaction. It emphasizes the importance of balanced chemical equations and mole ratios to determine the quantities of reactants and products. Various stoichiometric problems are presented, demonstrating how to convert between grams and moles using these ratios.