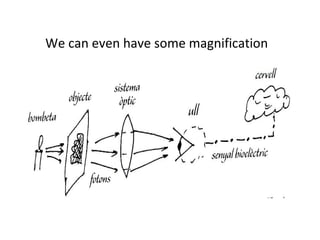
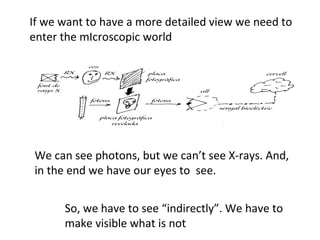
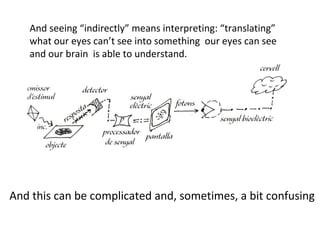
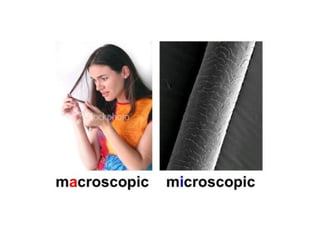
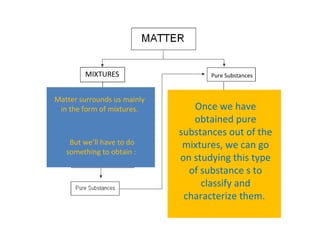
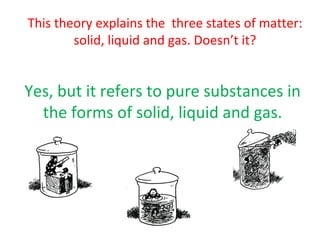
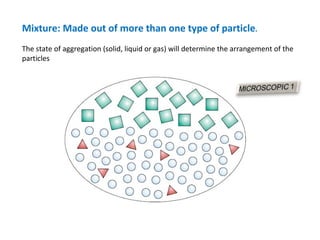
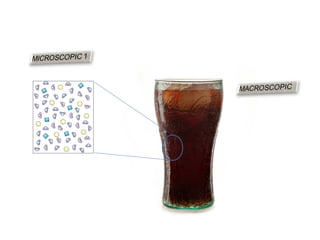
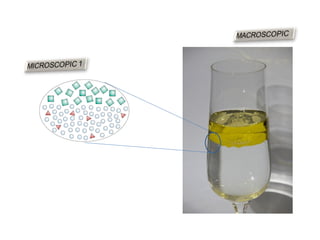
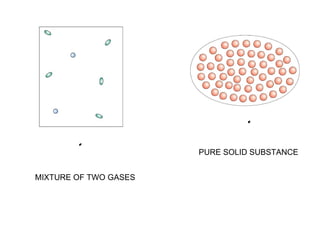
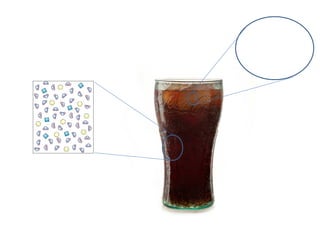
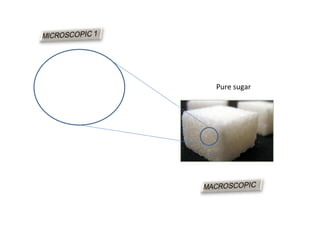
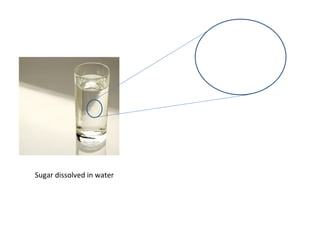
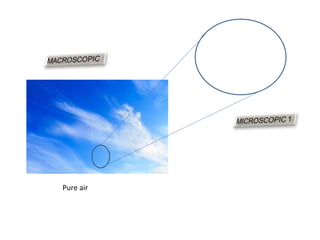
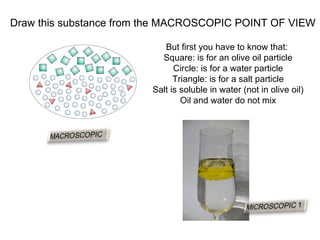
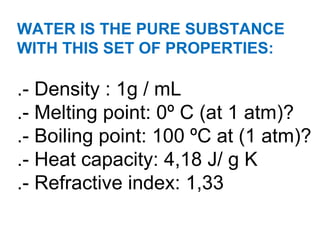
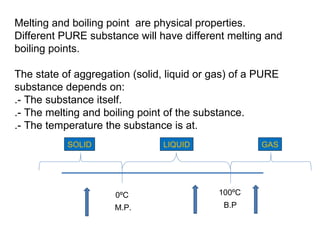
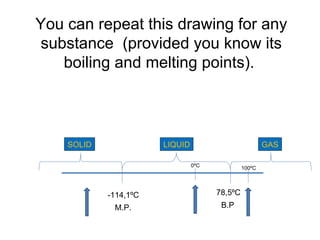
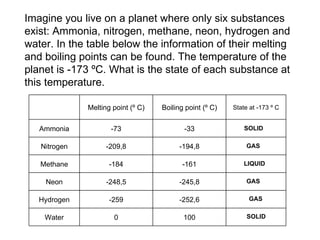
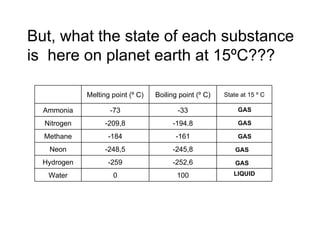
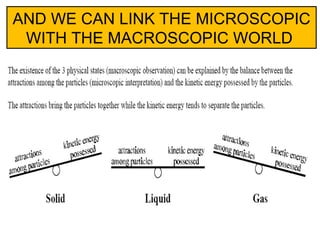
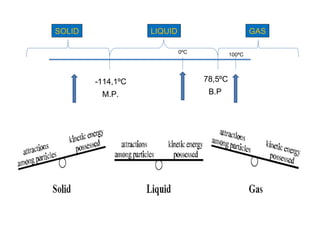
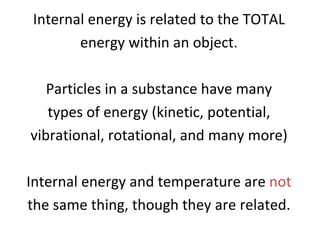
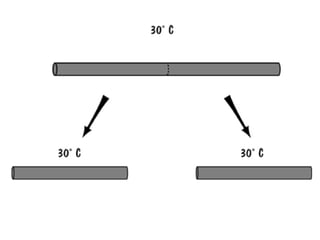
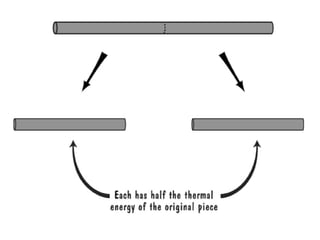
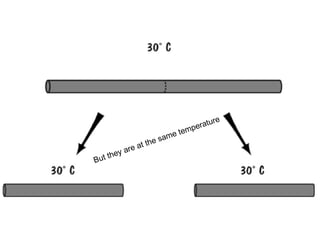
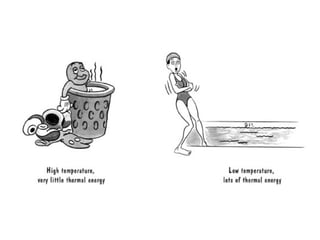
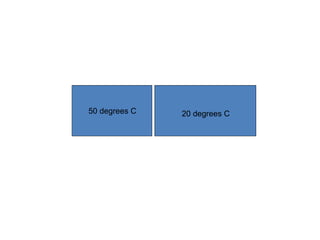
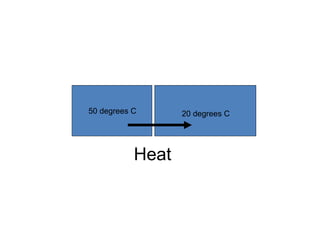
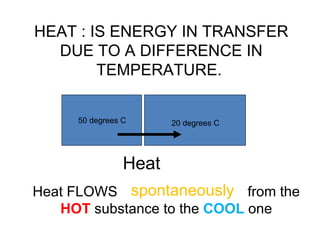
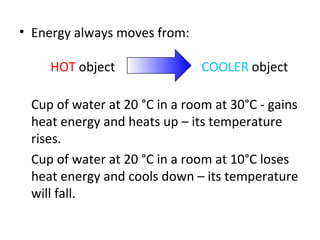
The document discusses the particle theory, which explains the properties and behaviors of matter by suggesting that all matter consists of small, constantly moving particles influenced by energy and attractive forces. It differentiates between pure substances and mixtures based on their particle composition and introduces concepts of macroscopic and microscopic perspectives, including temperature and state changes. Additionally, it explains how temperature relates to kinetic energy and how heat transfer occurs between substances at different temperatures.