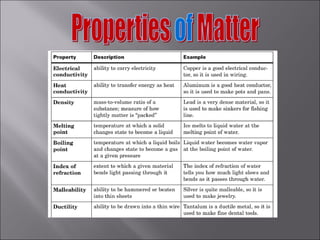
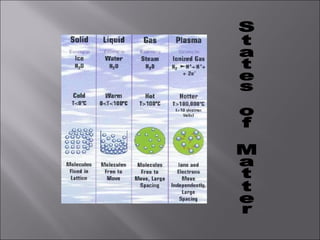
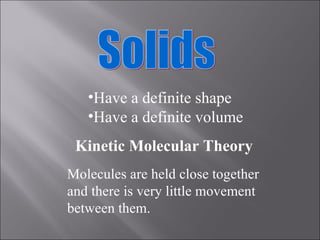
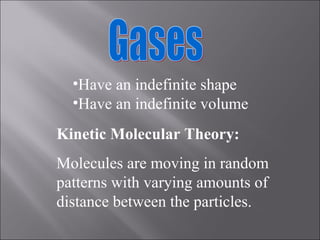
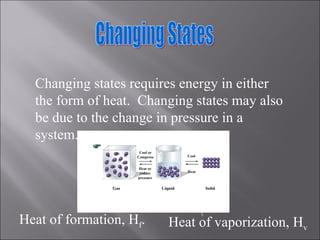
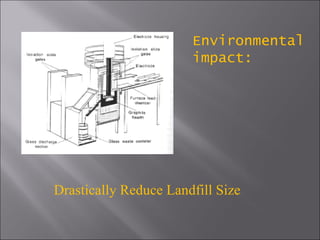
All matter can undergo physical and chemical changes. A physical change alters the appearance but not the chemical composition, such as water freezing. A chemical change forms new substances with different properties, like reactions with acids or bases. Substances have characteristic intensive properties that identify them and extensive properties that depend on amount.