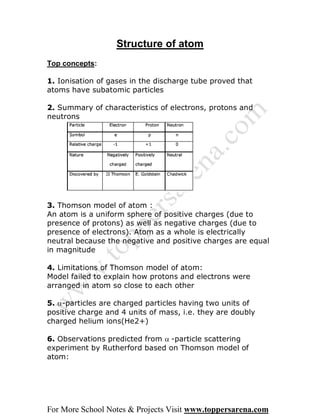
1. Rutherford's alpha particle scattering experiment showed that atoms have a small, dense nucleus containing positive charge and most of the atom's mass, with electrons orbiting the nucleus.
2. One out of every 12,000 alpha particles was deflected at a large angle, indicating the positive charge in an atom is concentrated in a very small nucleus.
3. Rutherford concluded atoms have a small, dense nucleus containing the atom's positive charge and most of its mass, with electrons orbiting the nucleus, resolving limitations of the previous Thomson model.