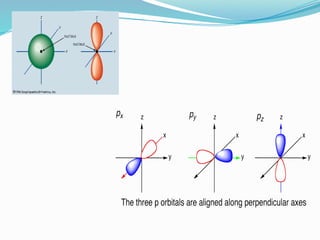
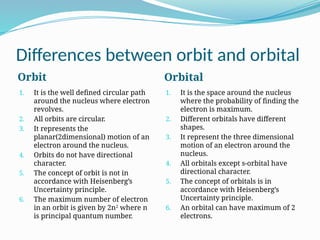
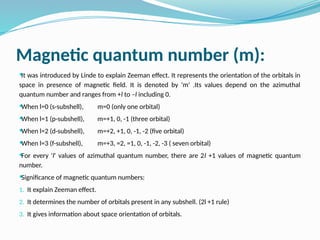
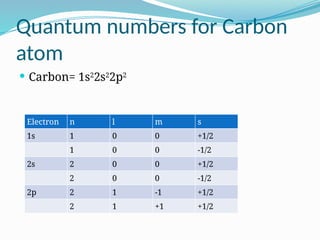
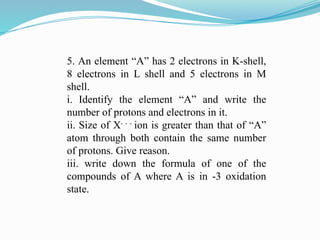
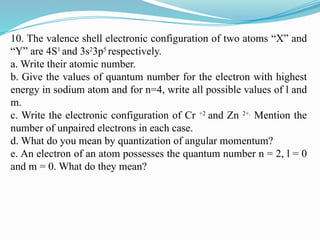
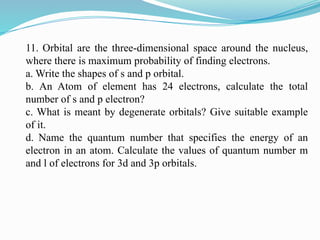
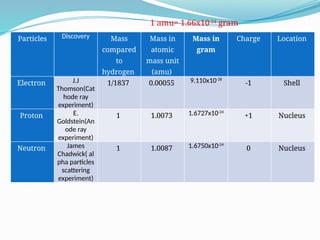
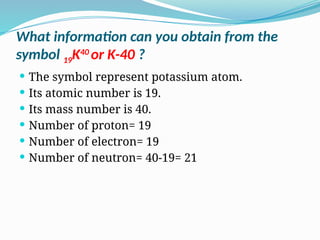
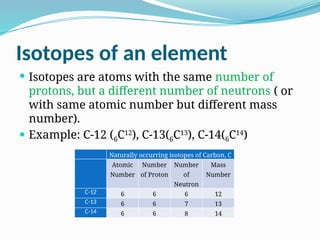
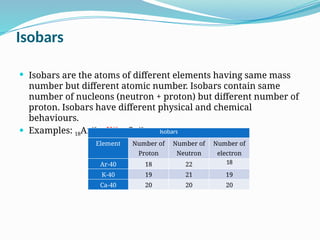
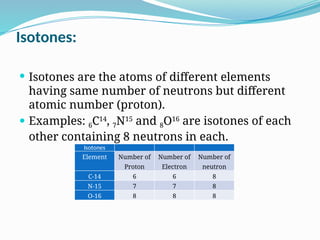
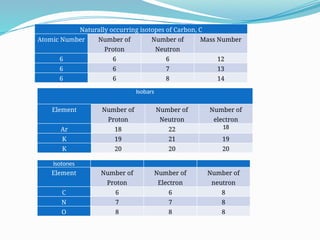
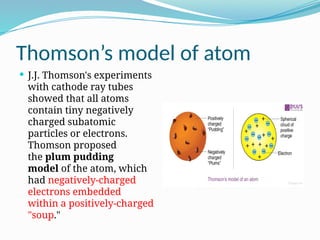
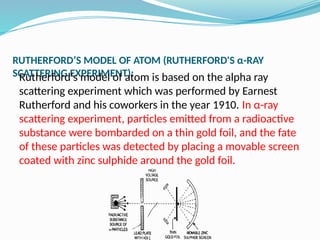
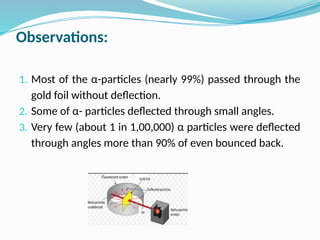
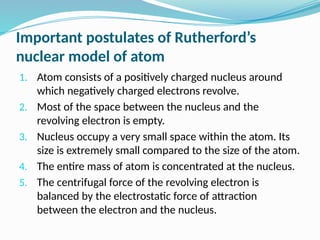
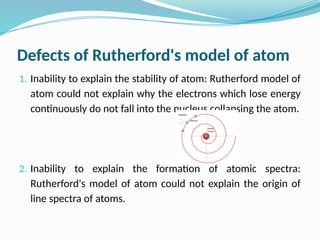
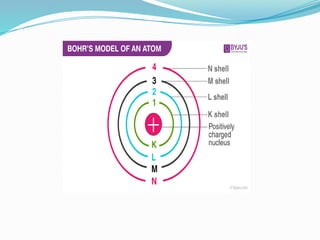
The document covers atomic structure, detailing the composition of atoms, including protons, neutrons, and electrons, and explaining key concepts like atomic number, mass number, isotopes, isobars, and isotones. It also discusses historical atomic models, specifically Thomson's plum pudding model, Rutherford's nuclear model, and Bohr's atomic theory, highlighting their postulates and limitations. Additionally, the document addresses the quantum mechanical model of the atom, including de Broglie's equation and the Heisenberg uncertainty principle.


















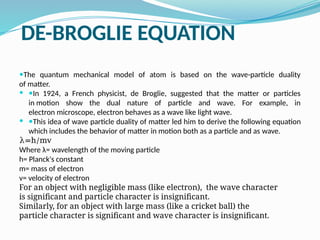
![Derivation of de Broglie’s equation
From Einstein equation;
E= mc2
…..(i)
where, m= mass of photon and c= velocity of light
From Planck’s equation;
E= hν …..(i)
where, h=planck’s constant and v= frequency of radiation
From equation 9i) and (ii)
mc2
= hν
mc2
= h(c/ ) [ν= c/ ]
= h/mc (for light)
= h/mv (for any material particle)
The equation is de-Broglie’s equation.
Therefore,
α(1/p) where mv= p= momentum
It follows that wave length of a particle is inversely proportional to its momentum.](https://image.slidesharecdn.com/atomicstructure2080-241130094135-7c35a777/85/Atomic-Structure_2080-pptxaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa-40-320.jpg)