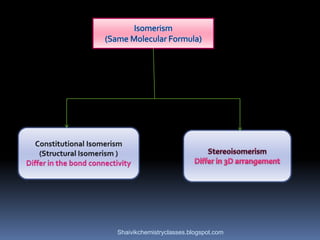
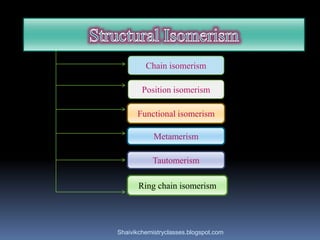
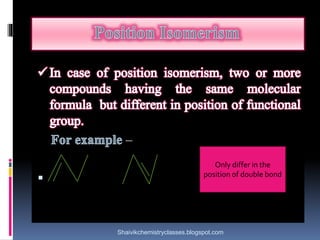
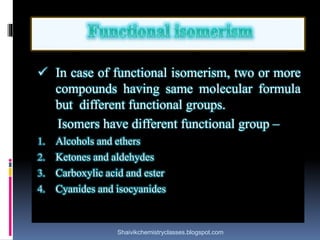
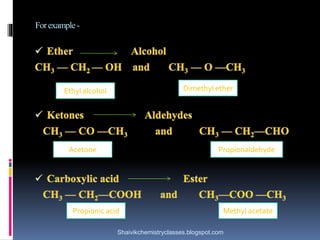
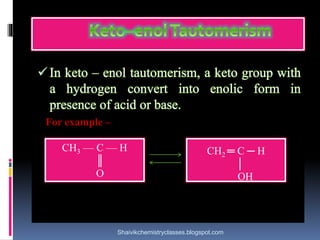
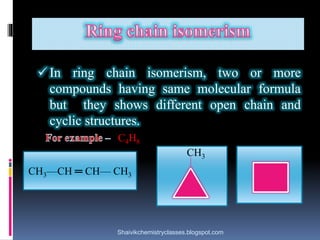
The document discusses various types of isomerism, including chain isomerism, functional isomerism, and tautomerism. It explains that chain isomers differ in the parent chain, functional isomers have different functional groups, and tautomers are isomers differing in proton position. It also outlines specific examples for each type and emphasizes the dynamic nature of tautomerism.