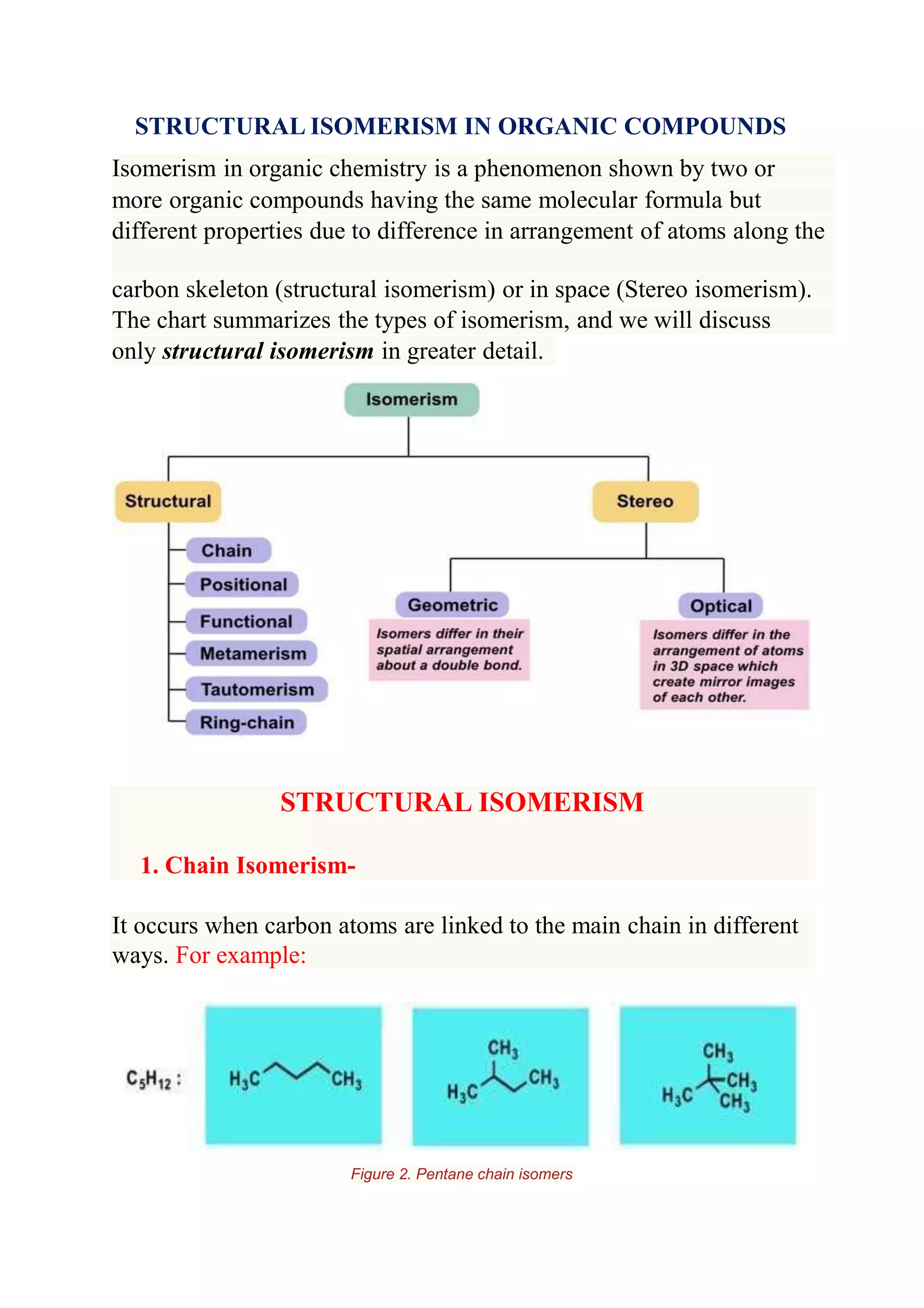
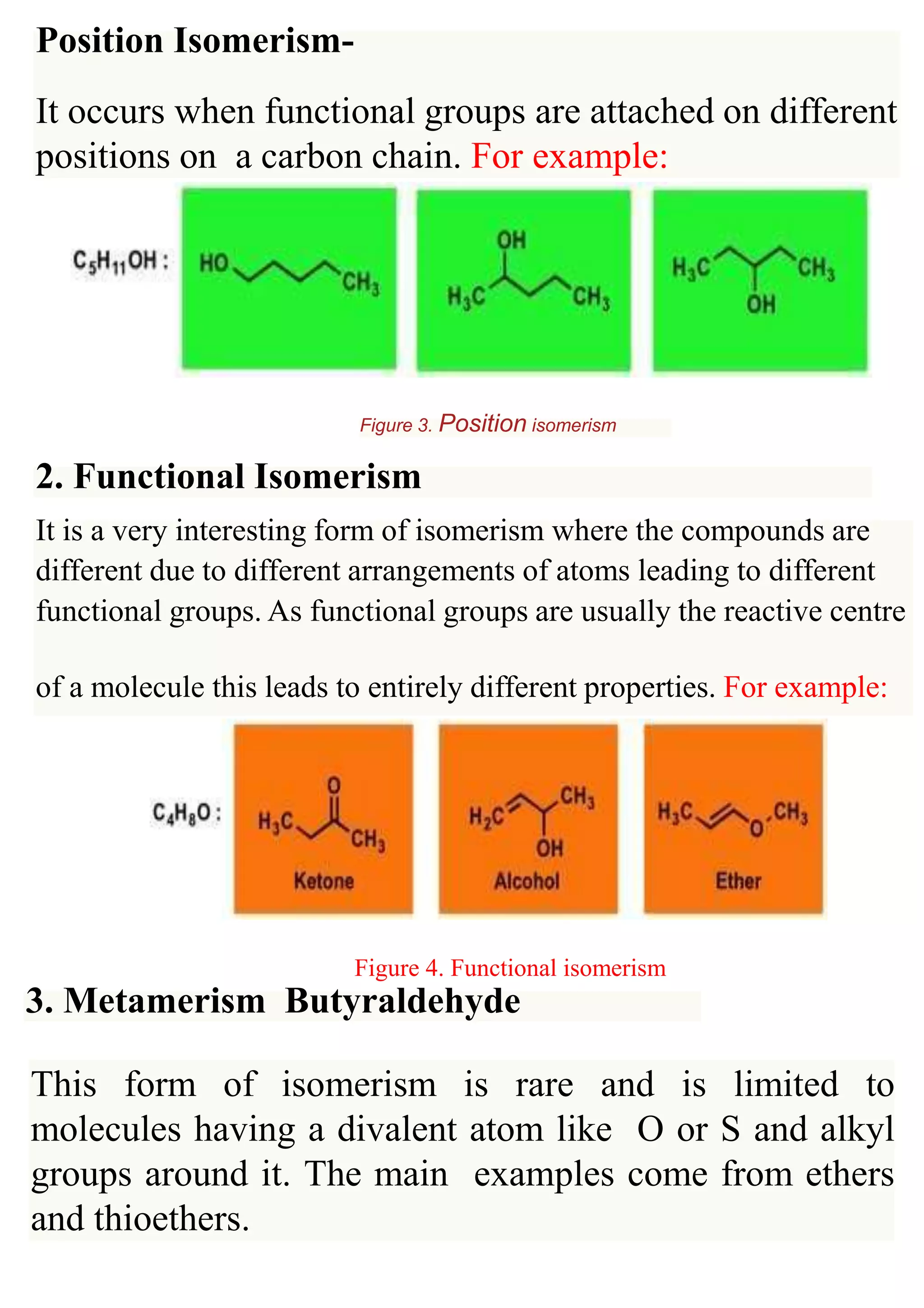
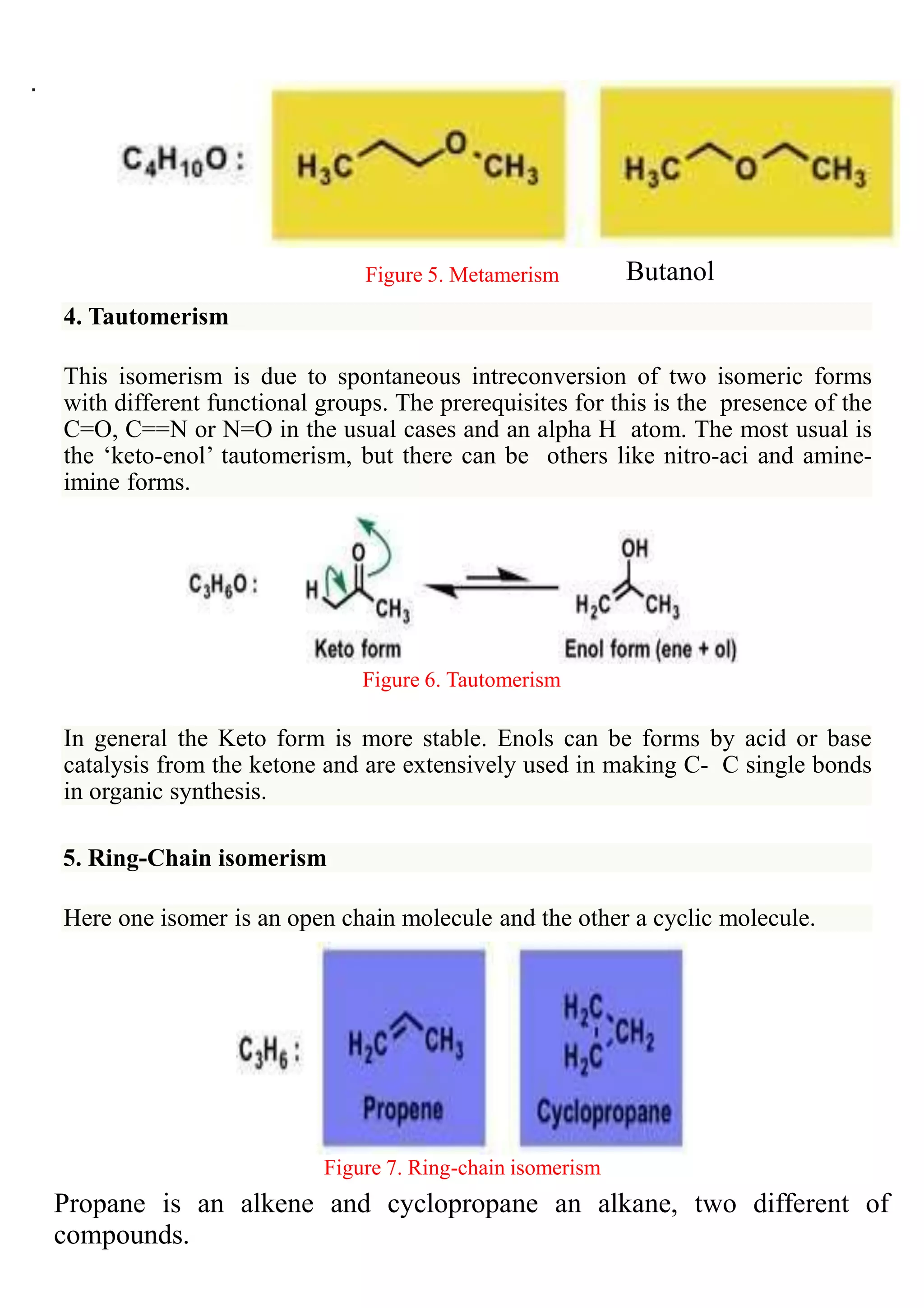
Structural isomerism occurs when compounds have the same molecular formula but different properties due to variations in how atoms are arranged along the carbon skeleton. There are several types of structural isomerism: chain isomerism, which involves differences in how carbon atoms are linked to the main chain; position isomerism, where functional groups are attached at different positions on a carbon chain; and functional isomerism, where different arrangements of atoms lead to different functional groups and properties. The document provides examples and figures to illustrate each type of structural isomerism.