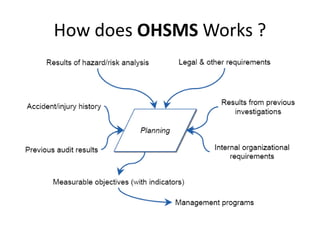
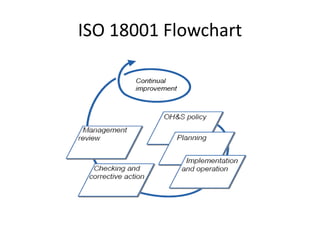
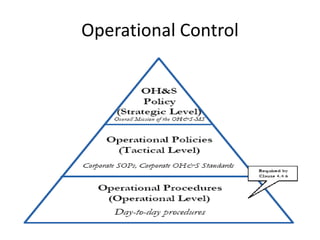
This document provides information about ISO 18001 and OHSAS 18001 standards for occupational health and safety management systems. It describes what OHSAS 18001 is, how it was developed, its key elements and requirements, benefits of certification, and compatibility with other ISO standards like ISO 9001 and ISO 14001. OHSAS 18001 provides a framework for organizations to systematically manage occupational health and safety risks and improve their performance in this area.