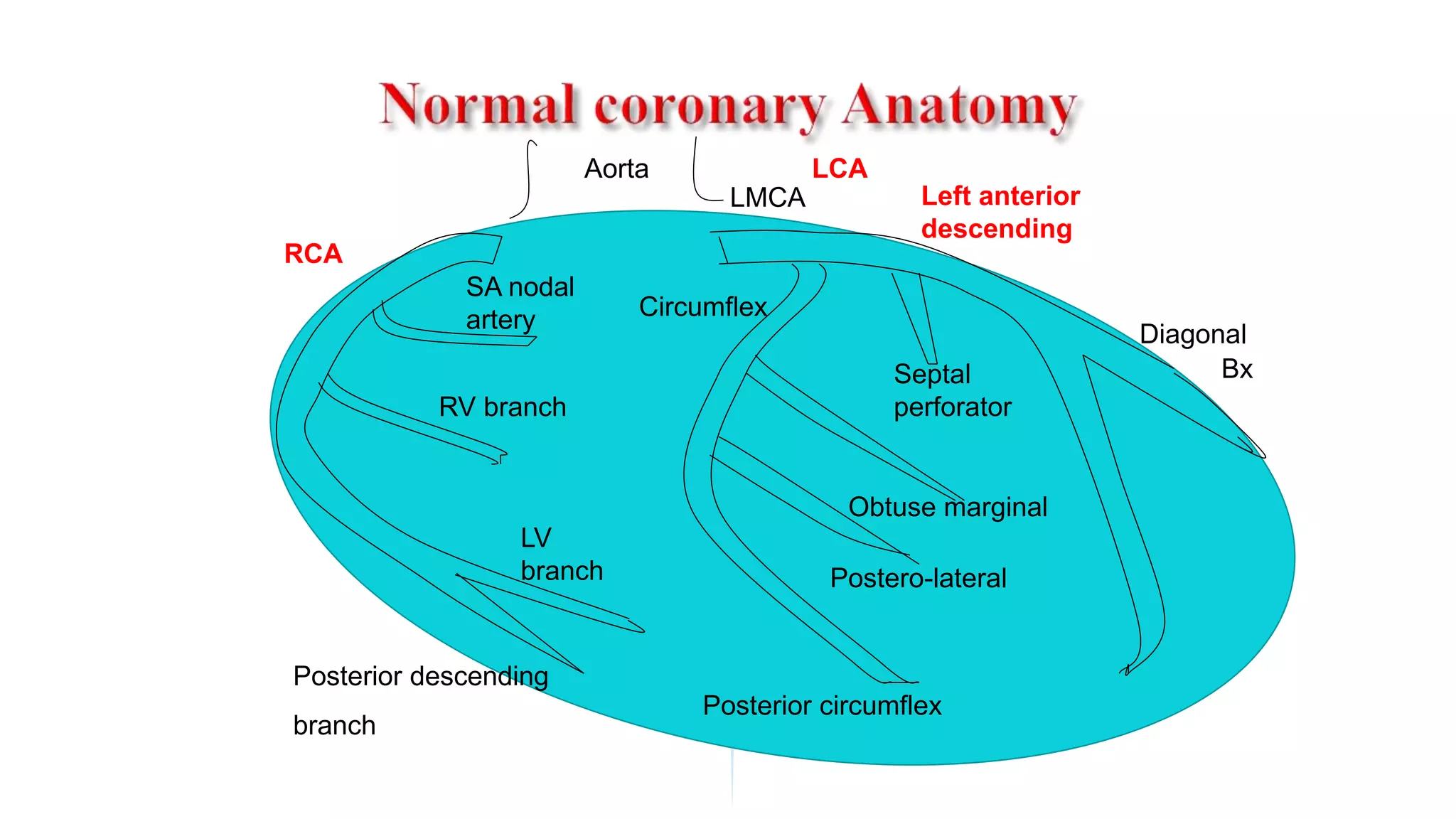
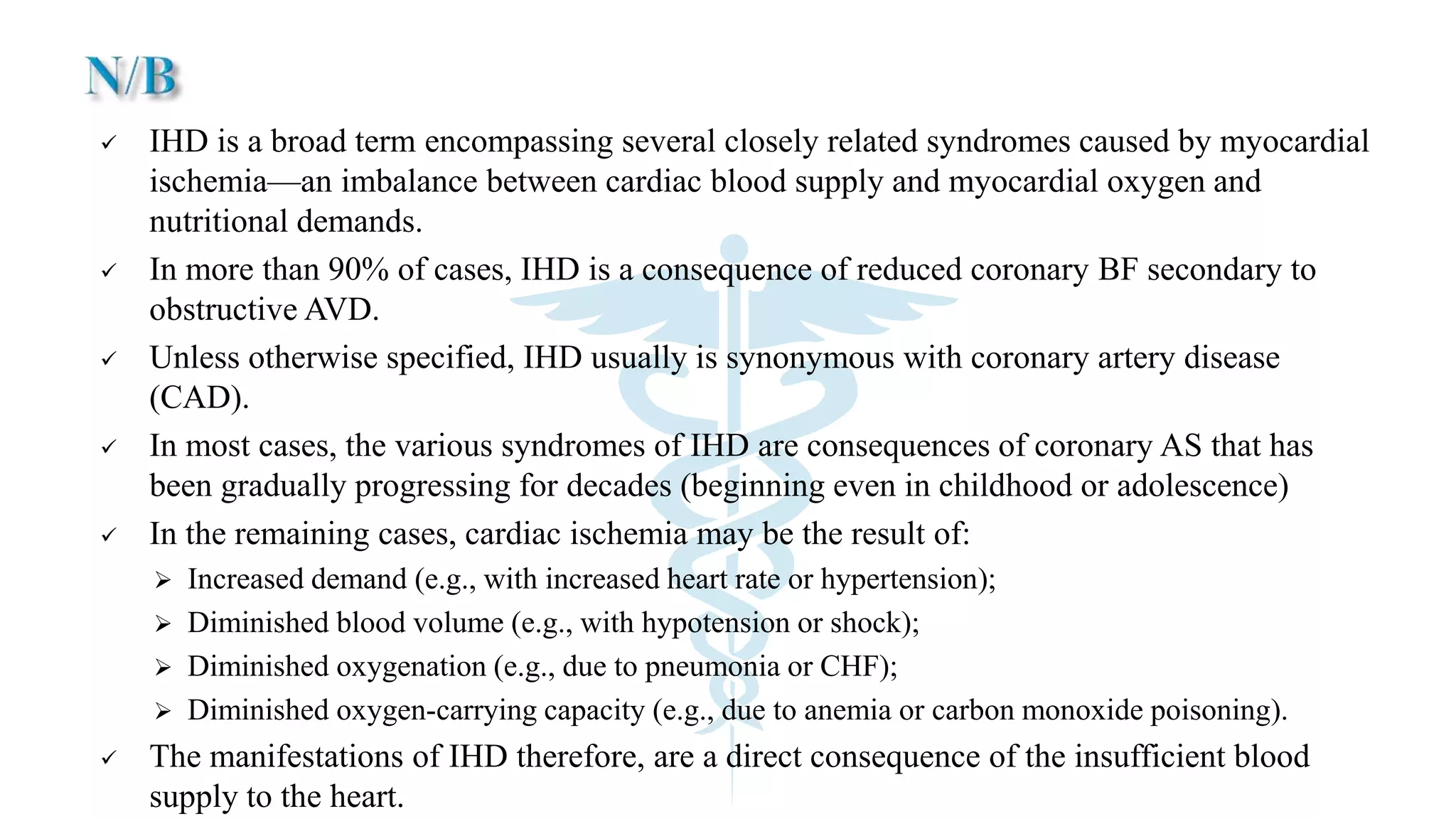
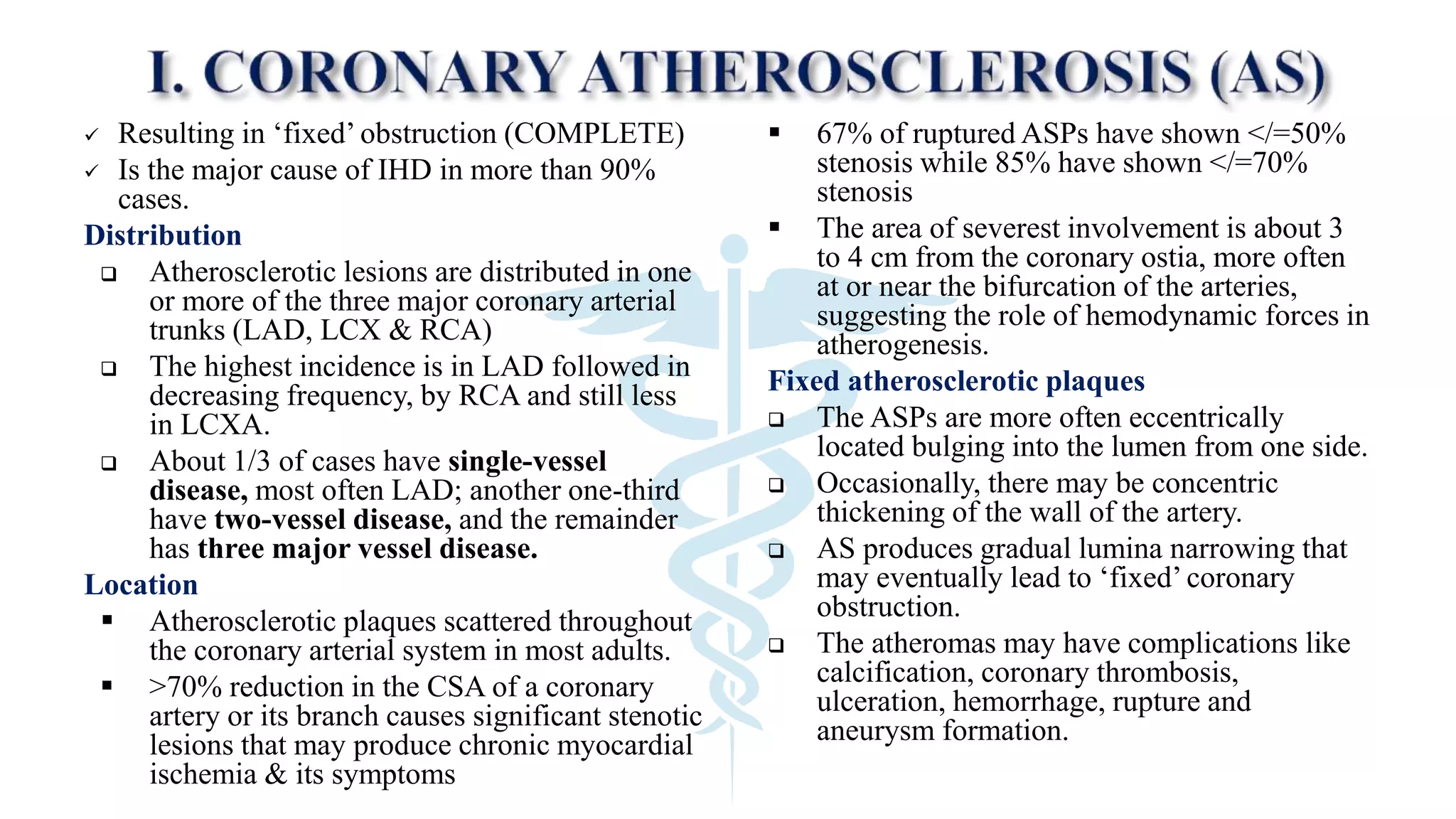
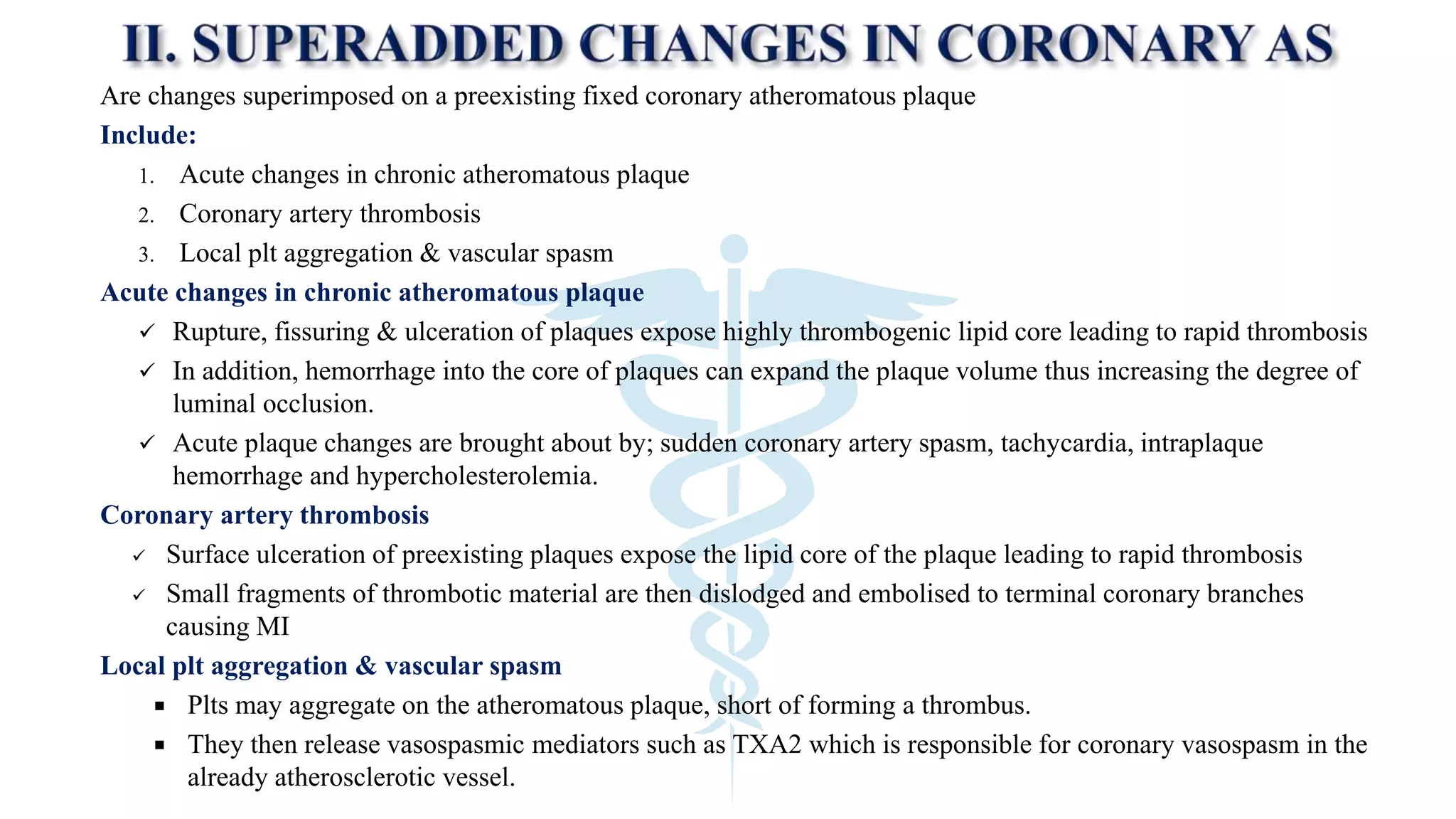
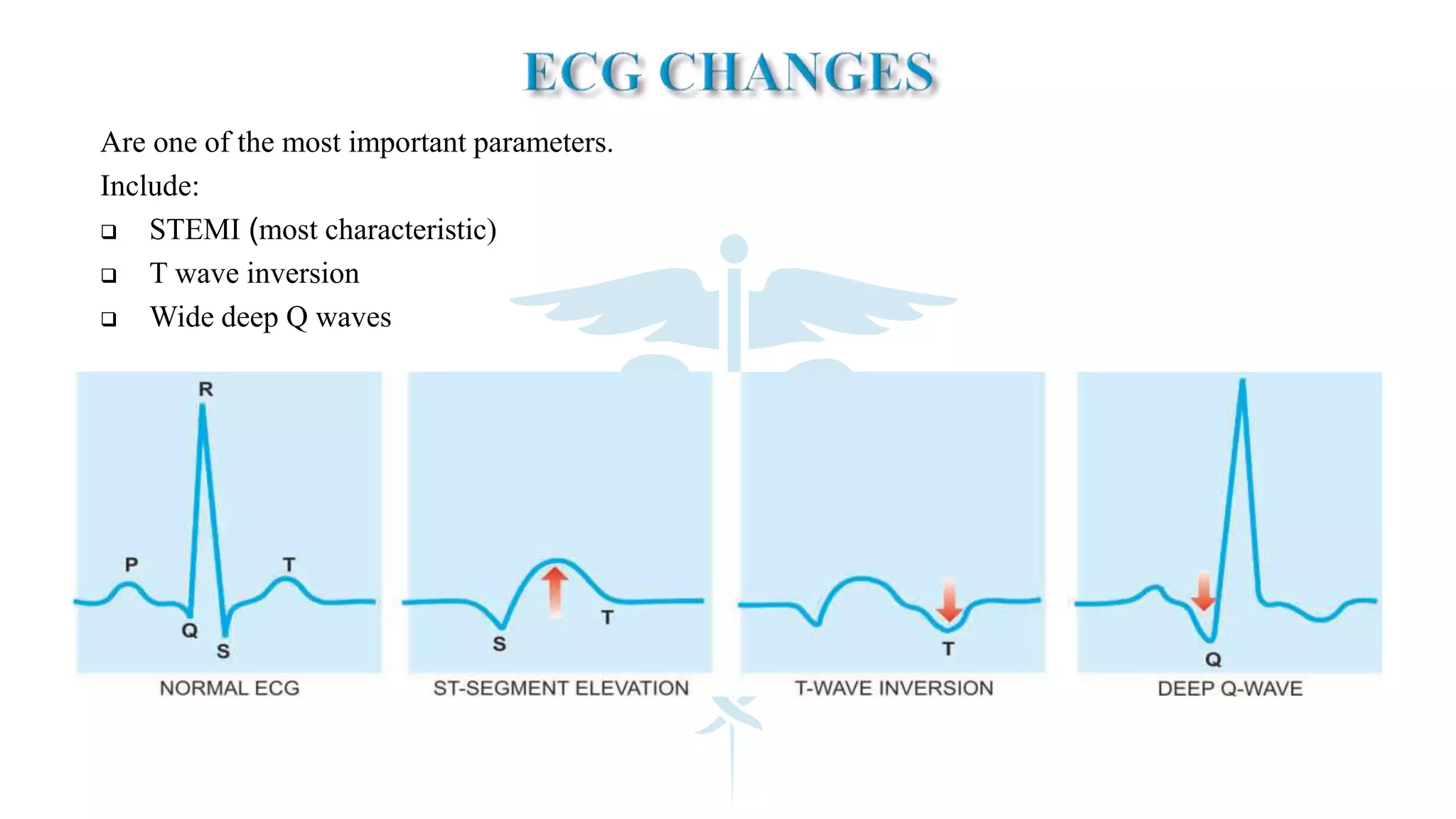
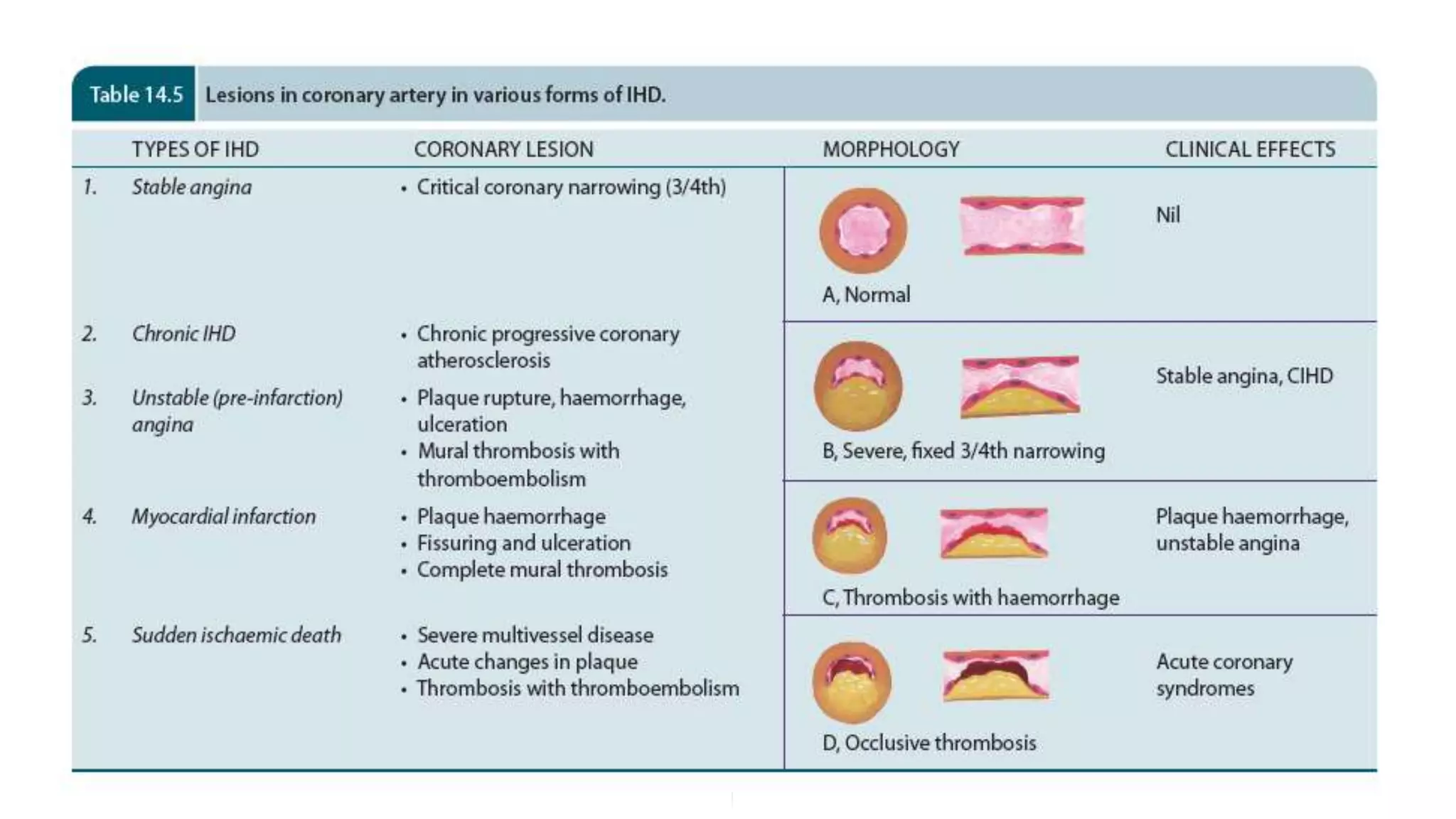
The document discusses coronary artery disease and myocardial infarction. It begins by describing normal coronary anatomy and physiology. It then defines ischemic heart disease and discusses its epidemiology and etiopathogenesis, focusing on atherosclerosis and other causes. It describes coronary syndromes like stable angina, unstable angina, and acute myocardial infarction. It provides details on pathogenesis, clinical features, and complications of myocardial infarction.