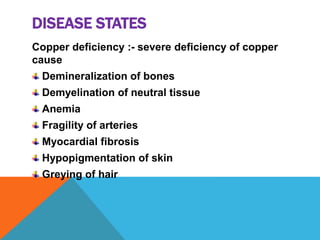
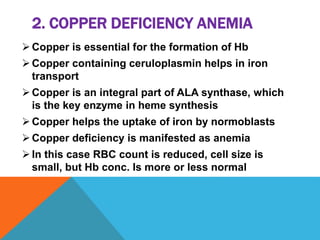
This document discusses copper, an essential mineral that has many functions in the body. It summarizes that copper is a soft metal with good electrical and heat conductivity. It is found in many foods and helps with iron absorption, enzyme activity, and heart health. The document outlines dietary requirements, absorption in the small intestine, transportation in the blood bound to proteins, and excretion levels. It also describes copper deficiency and several diseases related to abnormal copper metabolism like Wilson's disease and Menke's kinky hair syndrome. Common tests for measuring copper levels in serum are also presented.