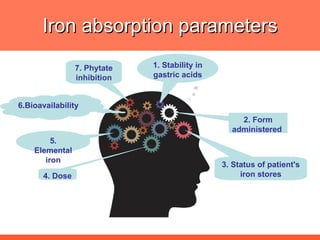
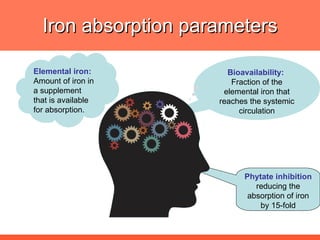
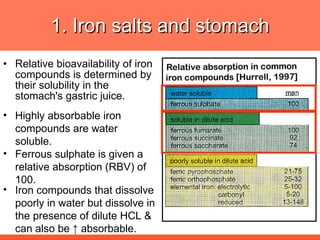
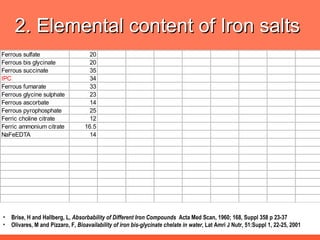
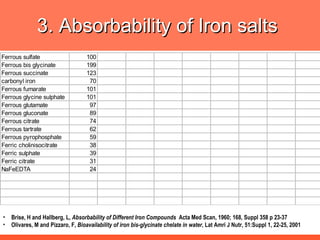
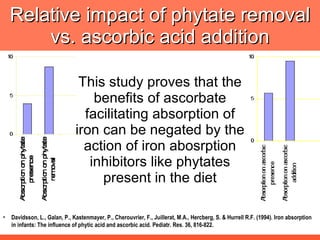
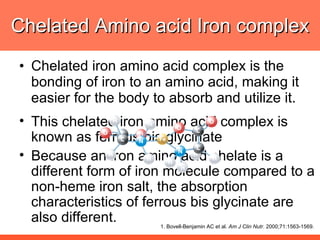
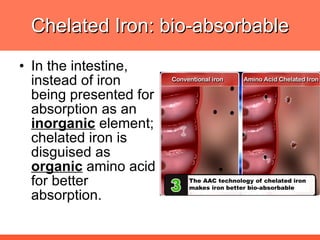
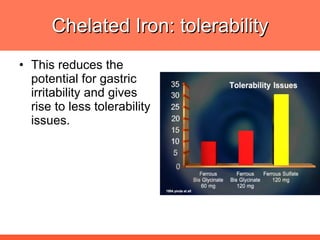
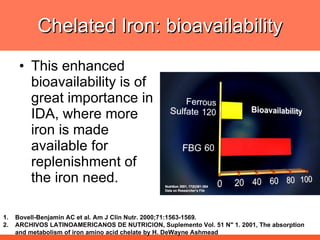
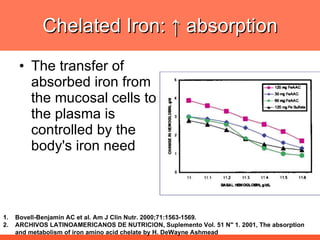
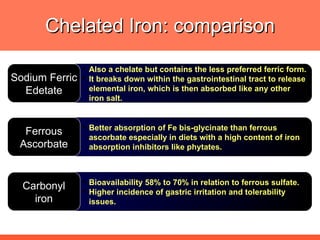
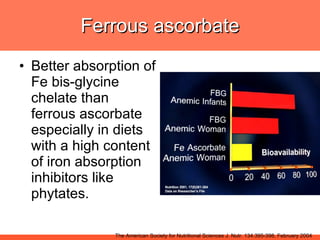
The document discusses newer aspects of iron supplementation. It summarizes that iron amino acid chelate, or ferrous bis glycinate, has advantages over other forms of iron supplementation, including being non-buffered in the stomach, non-precipitated in the intestine, not antagonized by phytates, and having superior and dependable bioavailability due to its unique chelate design, which potentially allows for smaller doses with fewer side effects. The document examines what is known, unknown, and needs to be known about different forms of iron supplementation and their absorption parameters.