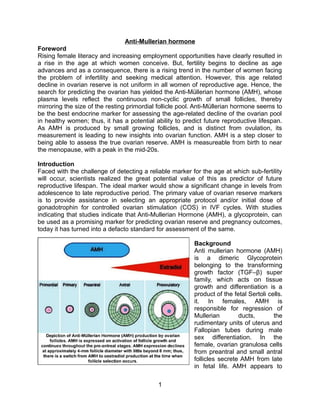
Rising age of motherhood has led to more women facing infertility issues. Anti-Mullerian hormone (AMH) levels can help predict ovarian reserve and remaining fertility as they reflect the number of follicles in the ovaries. AMH is produced from early fetal development through menopause, peaks in the mid-20s, and declines with age as ovarian reserve decreases. AMH levels are a stronger predictor of ovarian response to fertility treatments than age alone and can help determine initial fertility drug doses. AMH may also help diagnose conditions like polycystic ovarian syndrome and assess chemotherapy-induced damage to ovarian reserve.