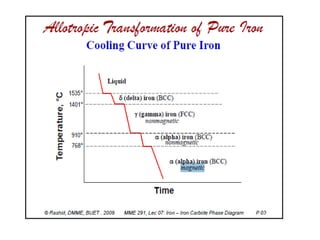
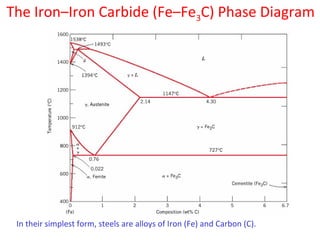
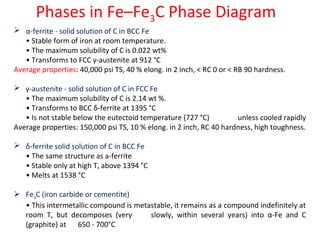
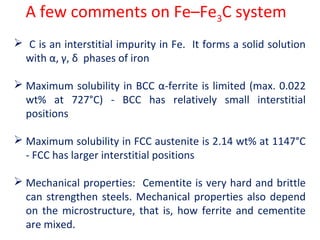
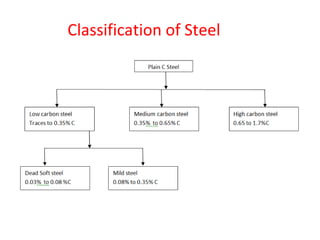
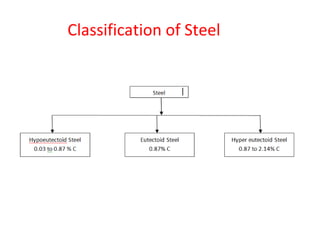
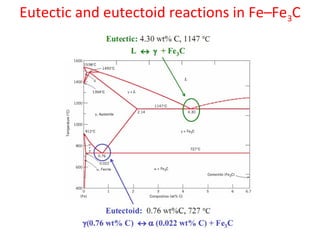
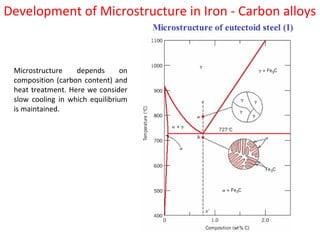
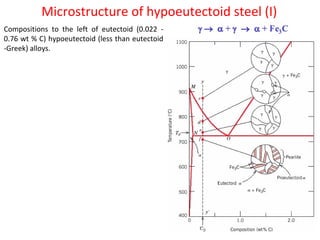
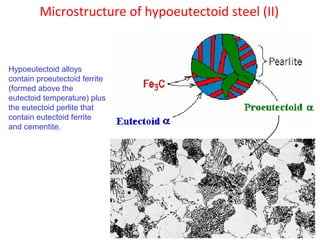
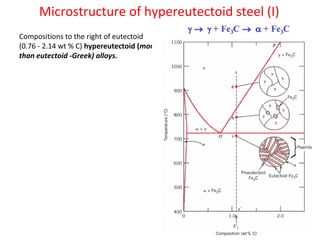
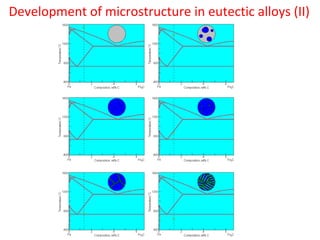
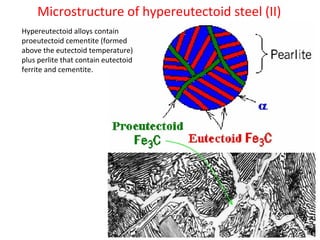
The document summarizes the iron-carbon phase diagram and the microstructures of steels with varying carbon compositions. It discusses the different phases in the diagram including α-ferrite, γ-austenite, δ-ferrite, and Fe3C. The solubility limits and properties of each phase are provided. Based on their carbon content, steels are classified as hypoeutectoid, eutectoid, or hypereutectoid and slowly cooled microstructures consist of combinations of ferrite and cementite arranged differently for each classification.