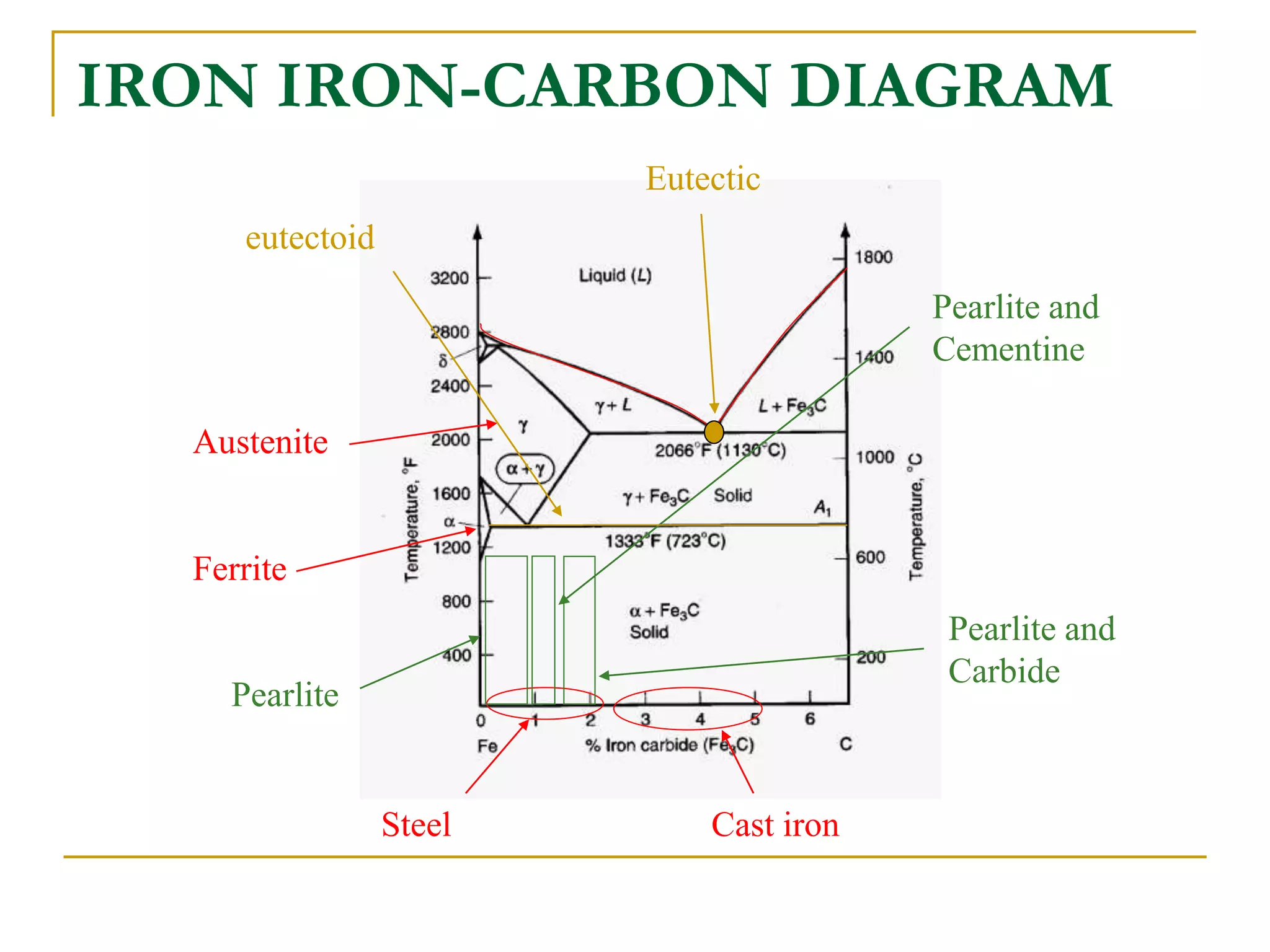
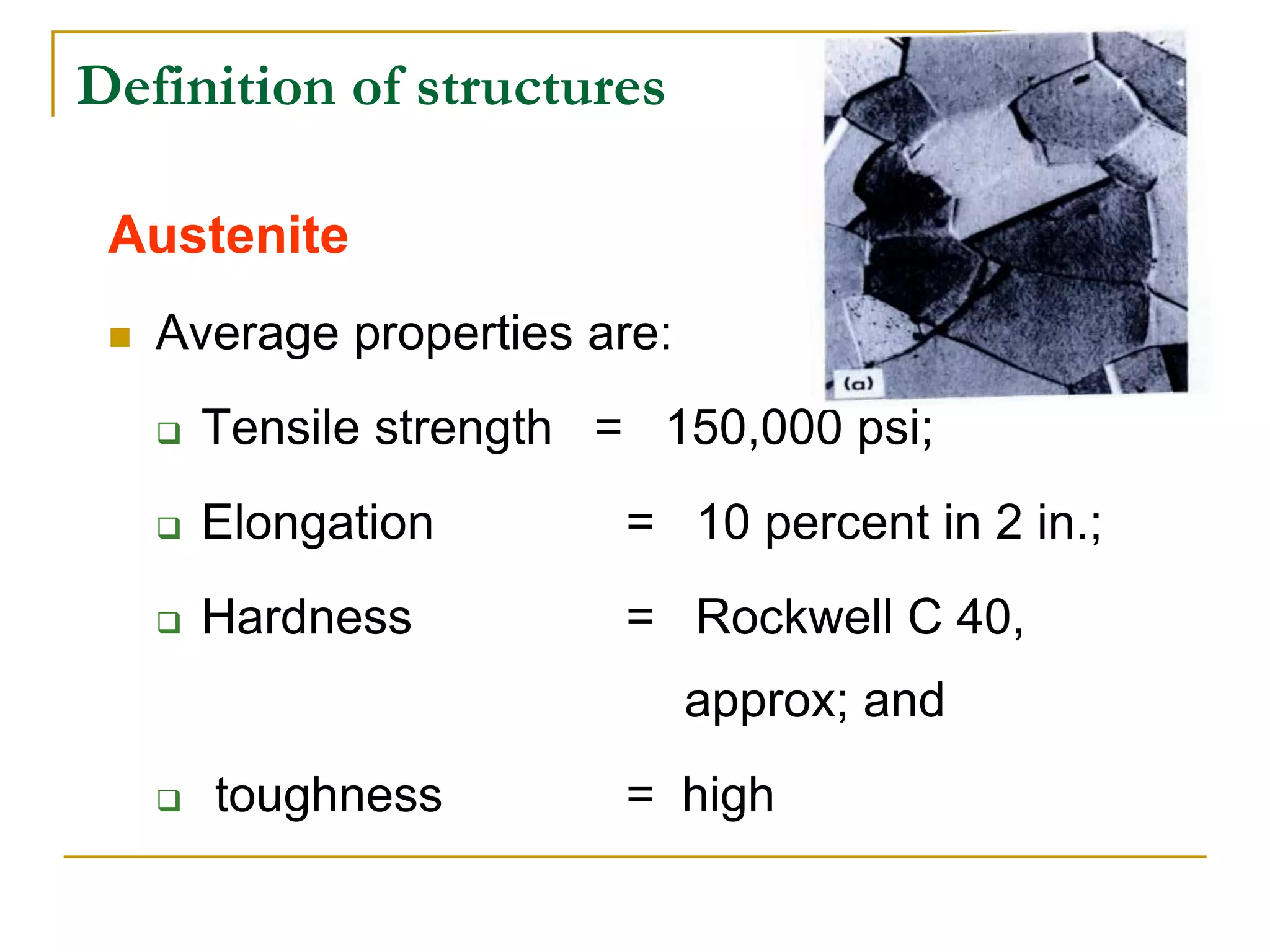
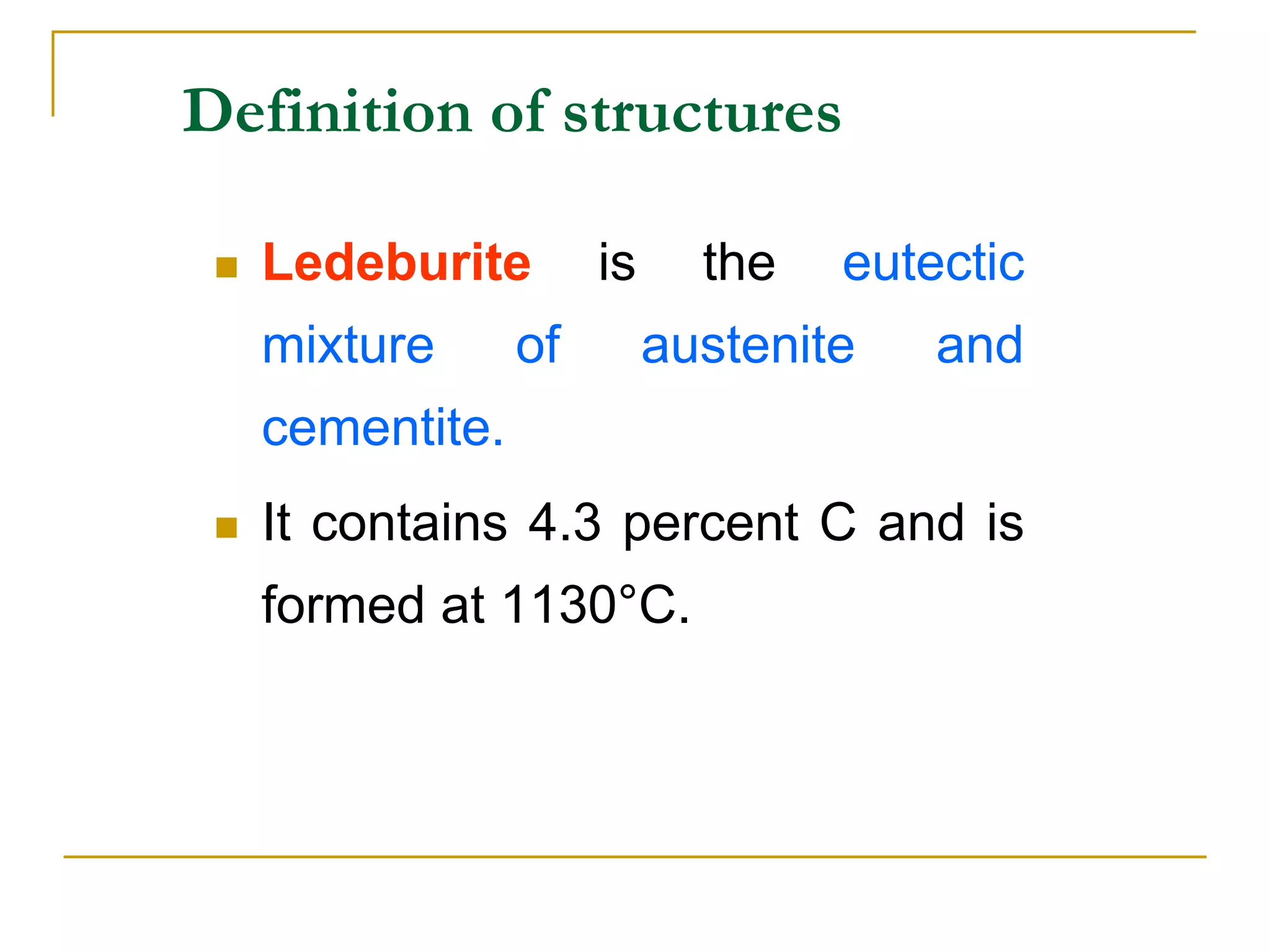
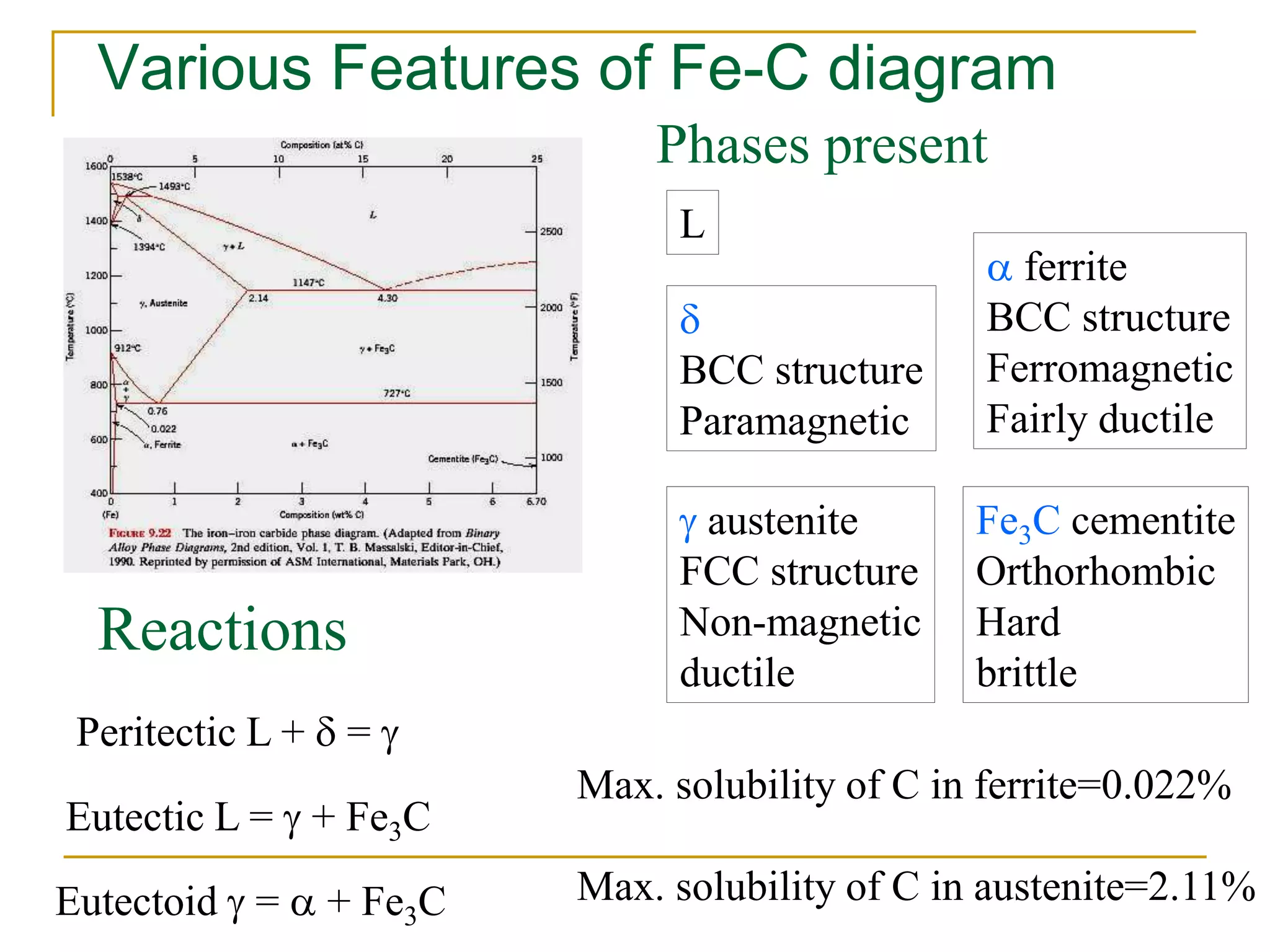
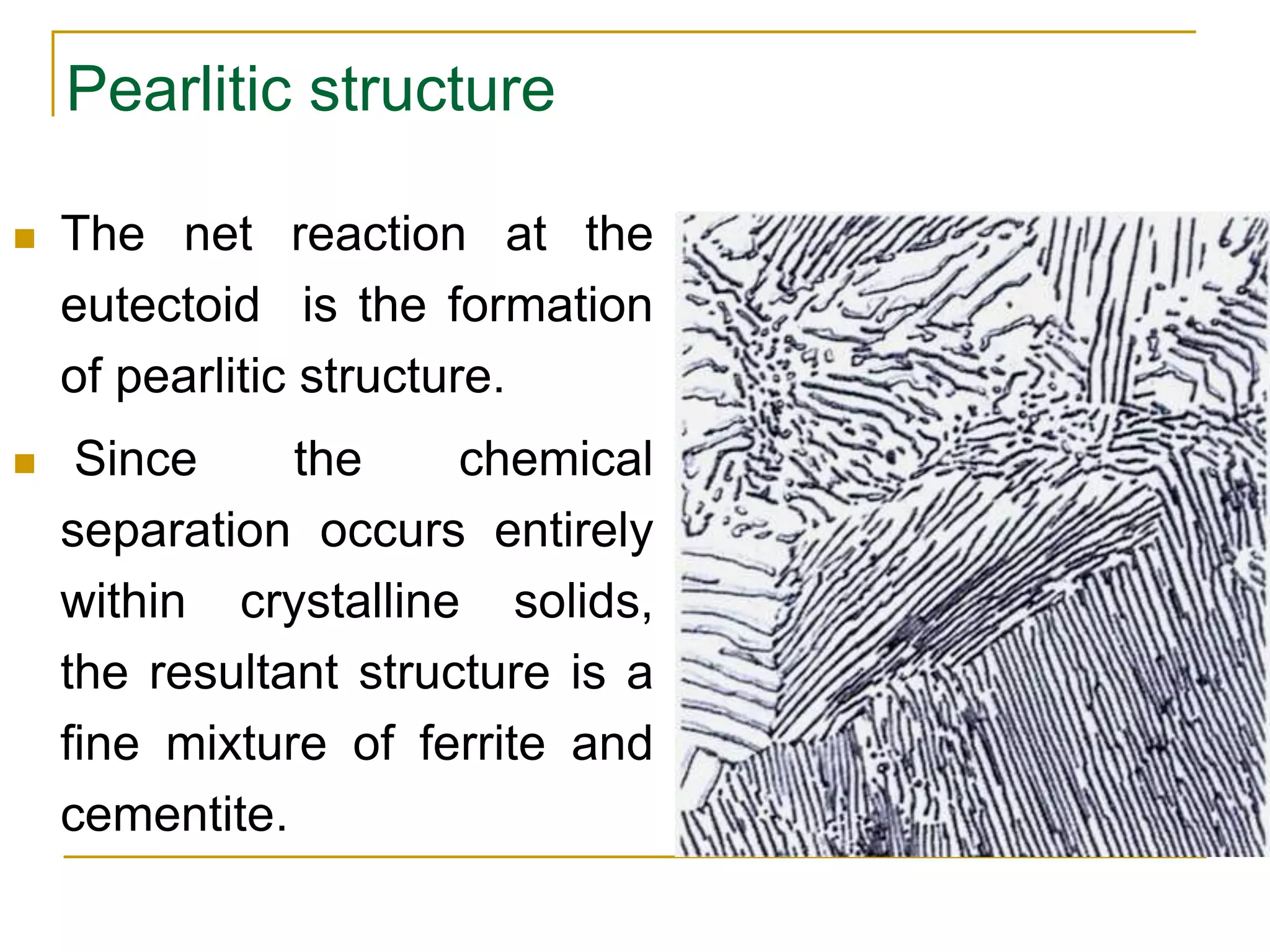
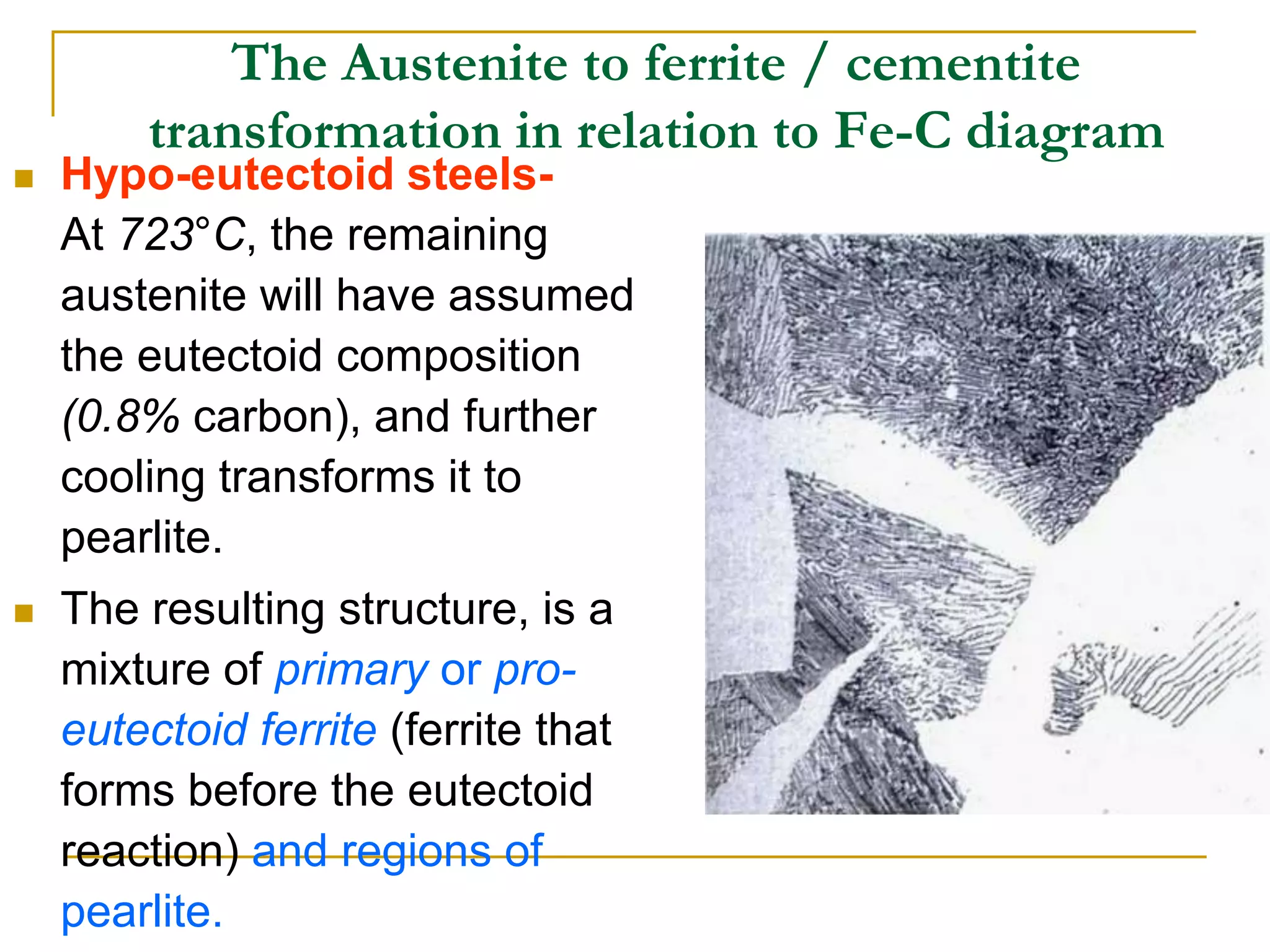
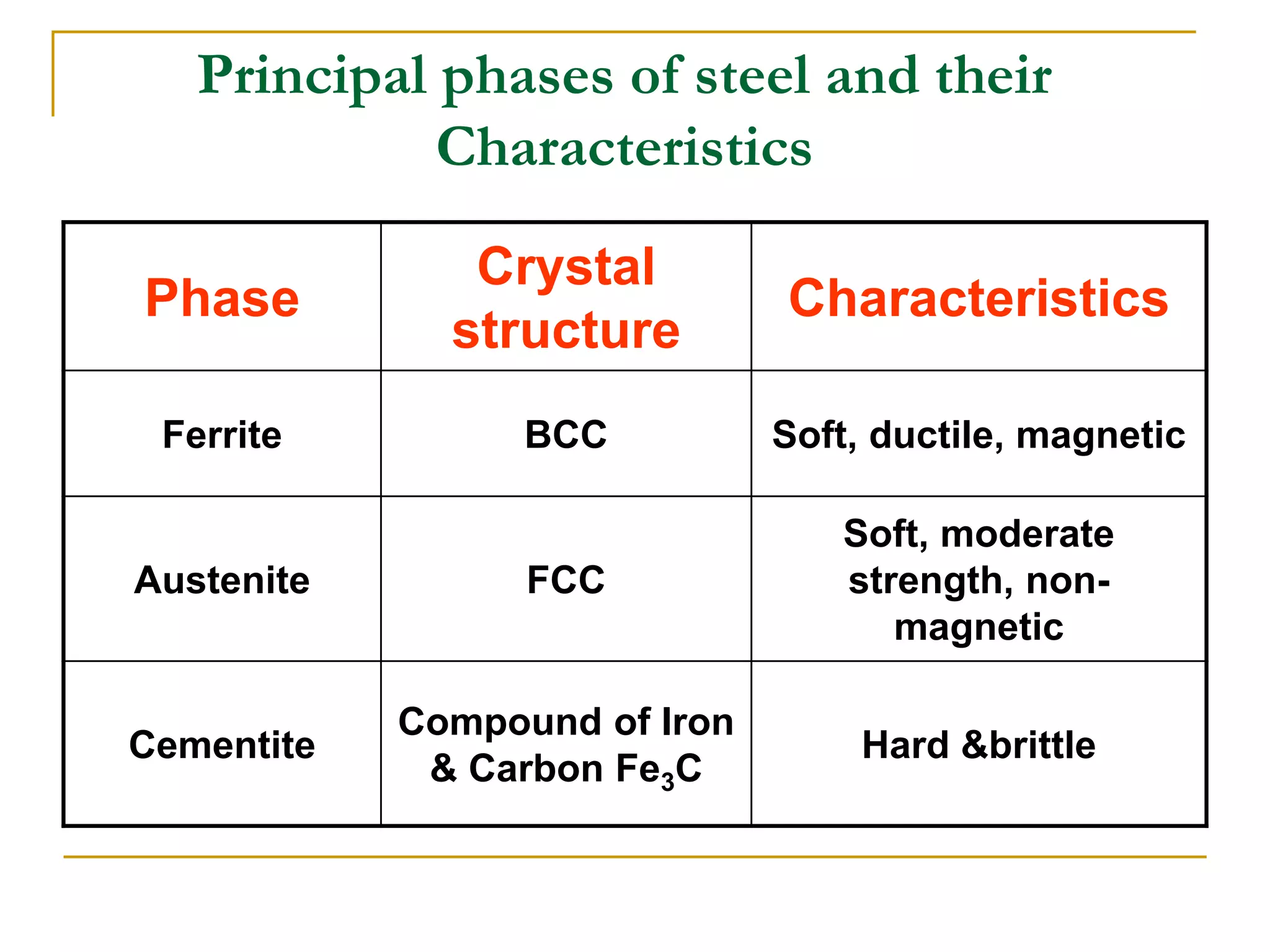
The document discusses the iron-carbon phase diagram, which maps the different crystal structures that form in iron-carbon alloys at various temperatures and carbon percentages. It defines various structures including ferrite, austenite, pearlite, and cementite. The phase diagram shows three main reactions - the peritectic, eutectic, and eutectoid reactions. Based on their carbon percentage, steels can form different microstructures like ferrite-pearlite or cementite-pearlite when cooled from austenite. The diagram is important for understanding steel heat treatments and tailoring mechanical properties.