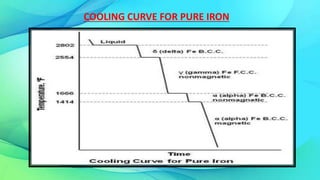
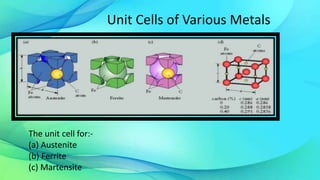
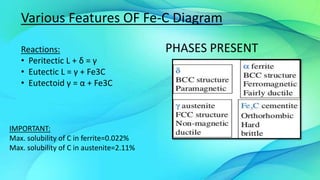
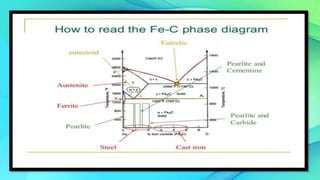
The document discusses the iron-carbon equilibrium diagram, focusing on the phases of steel and cast iron up to 6.67% carbon, including key structures such as ferrite, austenite, and cementite. It details the properties of iron and its allotropy, as well as the significance of temperature in phase changes and solubility limits of carbon in various iron forms. Furthermore, it outlines important reactions within the diagram and applications for tailoring steel properties and structural comparisons in metallurgy.