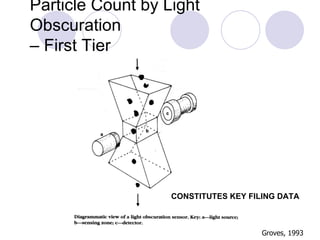
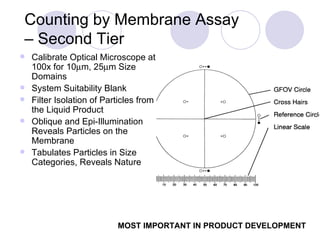
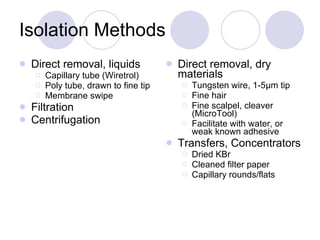
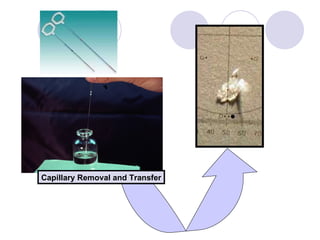
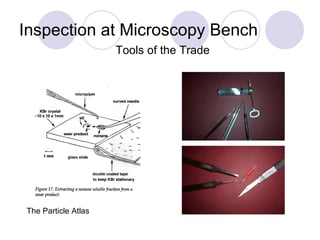
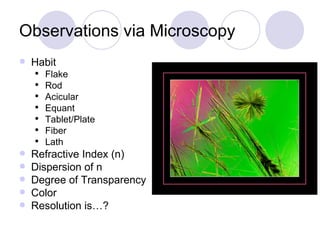
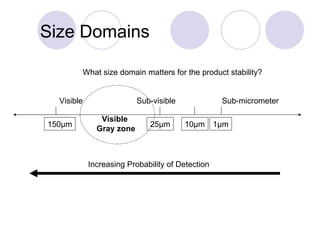
Visual inspection is an important first step in the particle identification process for pharmaceutical liquids and presentations. It provides an overview of product characteristics and defects that can be connected to particle content. Membrane microscopic counting is a key technique for quantifying particulate matter at various size ranges. Proper isolation, characterization, and identification of particles is essential to determine their origin and ensure product quality and stability.





![Compendial Requirements Robust, Safe, Sterile, Pure and Effective USP Chapter <1> Injections USP Chapter <788> Injections/<789> Ophthalmic Products USP/EP/JP have Harmonized <788> PM Testing Pharm. Forum re: Chapter <788>: IM and Sub-Q must meet <788> Pharm. Forum 35[3] May-June 2009, page 628 Radiopharmaceuticals are exempt from <788> limits Parenteral products for which labeling specifies use of a final filter are exempt from <788>, provided scientific data are available to justify the exemption. (do your homework). Irrigating solutions are exempt.](https://image.slidesharecdn.com/pdavisualinspection2009aldrich-12760394137902-phpapp01/85/Pda-Visual-Inspection-2009-Aldrich-11-320.jpg)