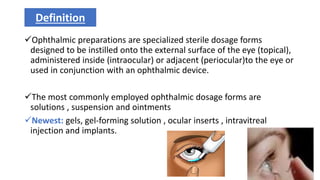
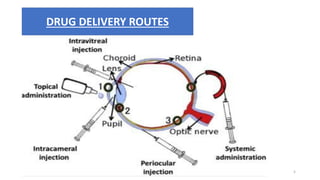
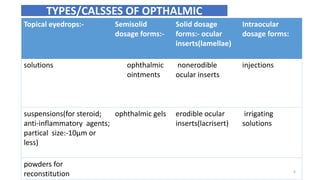
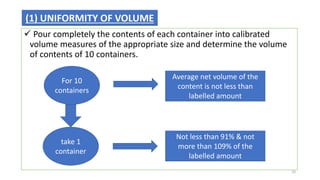
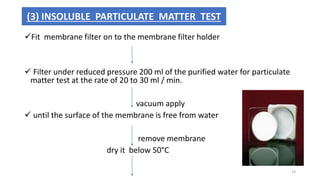
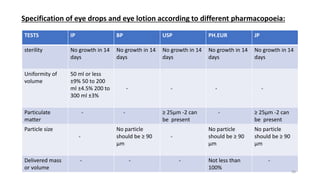
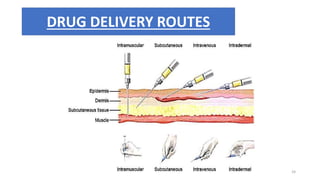
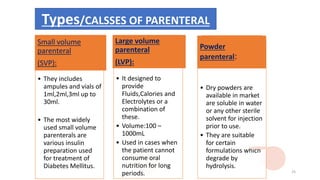
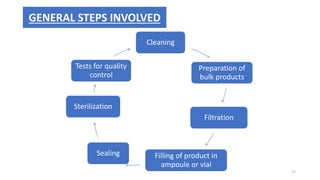
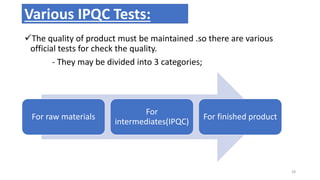
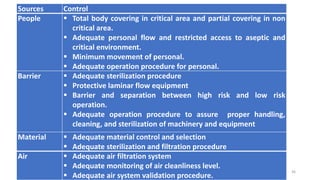
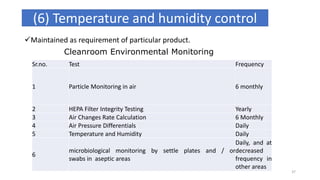
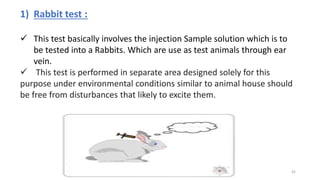
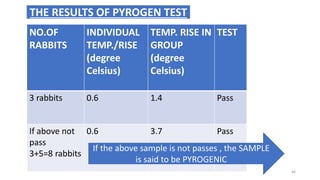
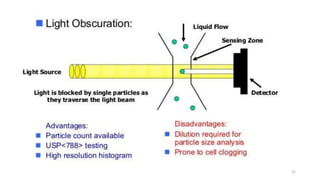
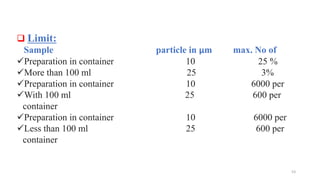
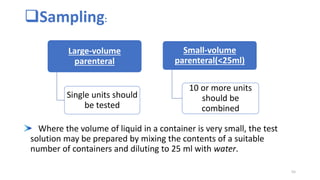
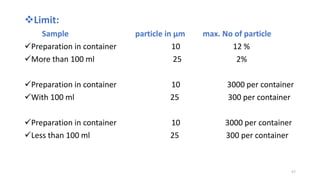
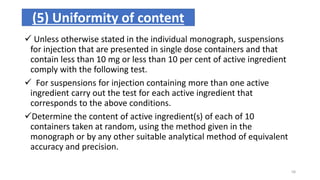
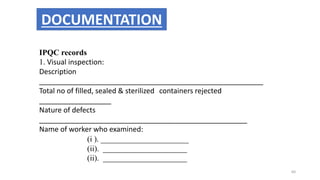
This document provides information on in-process quality control (IPQC) tests for ophthalmic and parenteral dosage forms. It defines ophthalmic and parenteral preparations and lists their common types. For ophthalmics, tests include sterility, uniformity of volume, particulate matter, particle size, and uniformity of weight. For parenterals, tests check content, pH, clarity, environmental controls and sterility. The document outlines procedures for sterility testing using direct transfer and membrane filtration methods and the rabbit pyrogen test.