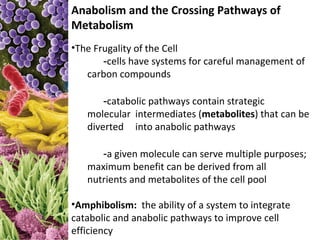
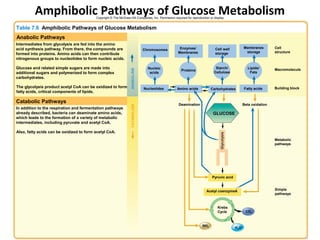
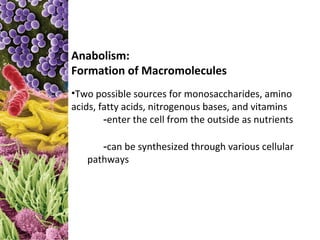
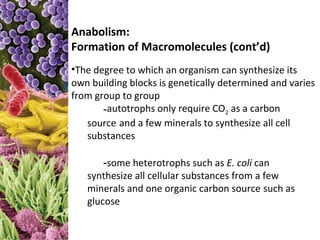
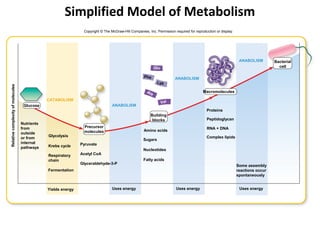
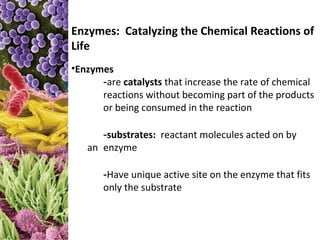
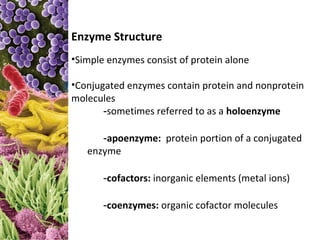
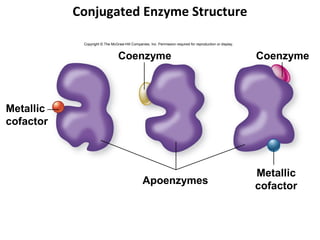
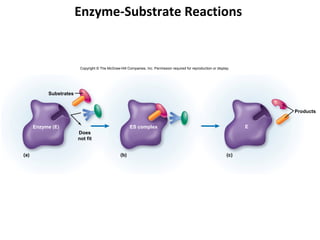
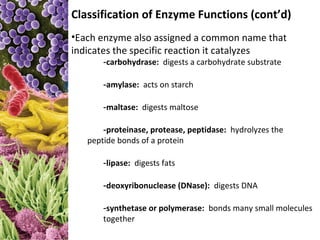
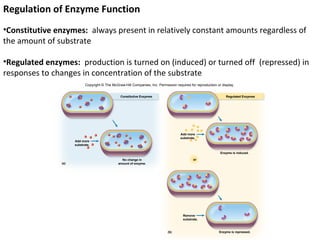
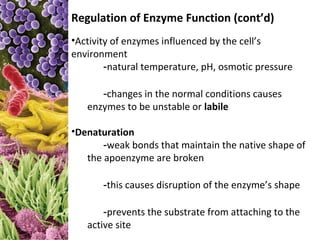
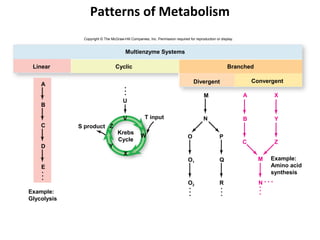
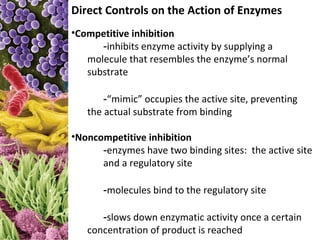
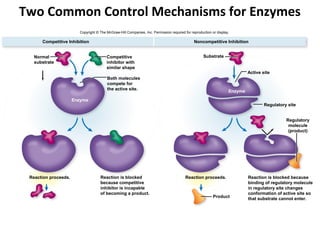
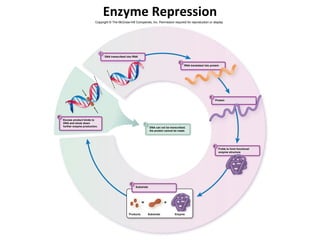
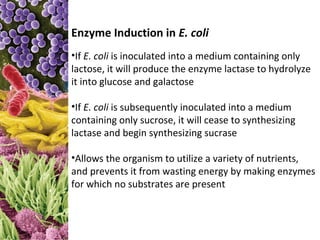
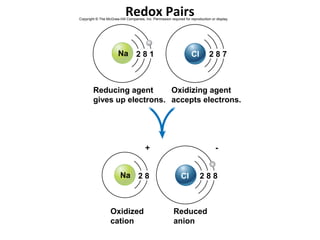
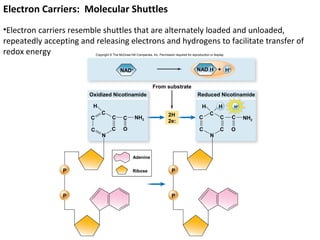
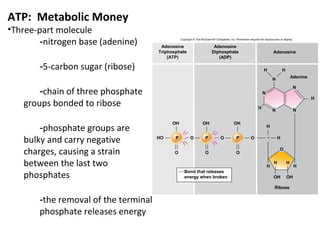
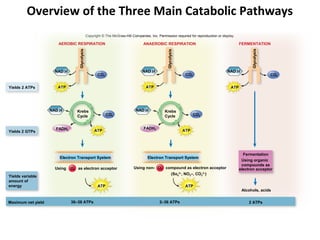
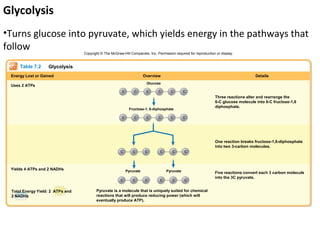
This document provides information about microbial metabolism and enzymes. It begins with learning outcomes about metabolism, enzymes, and metabolic pathways. It then defines metabolism as all chemical reactions in the cell, and defines anabolism as building reactions that require energy, and catabolism as energy-releasing breakdown reactions. Enzymes are described as catalysts with specific active sites that lower reaction activation energy. Regulation of enzymes through feedback inhibition or induction in response to substrates is covered. Electron carriers like NADH and FAD that facilitate energy transfer in redox reactions are also discussed. ATP is described as the main energy currency molecule in cells.














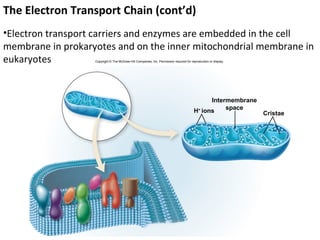
![The Respiratory (Electron Transport) Chain
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
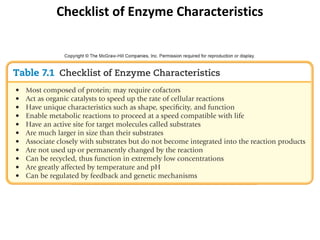
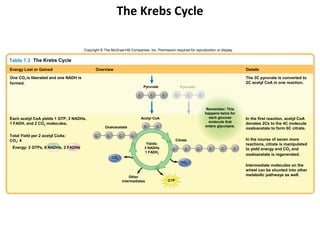
Table 7.4 The Respiratory (Electron Transport) Chain
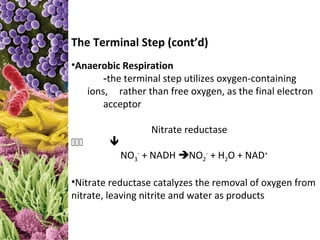
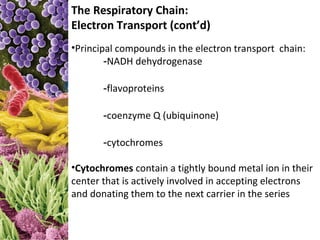
Reduced carriers (NADH, FADH) transfer electrons and H+ to first
electron carrier in chain: NADH dehydrogenase.
These are then sequentially transferred to the next four to six
carriers with progressively more positive reduction potentials.
The carriers are called cytochromes. The number of carriers varies,
depending on the bacterium.
Simultaneous with the reduction of the electron carriers,
protons are moved to the outside of the membrane, creating a
concentration gradient (more protons outside than inside the
cell). The extracellular space becomes more positively charged
and more acidic than the intracellular space. This condition
H+ creates the proton motive force, by which protons flow down the
H+ concentration gradient through the ATP synthase embedded in the
H+ membrane. This results in the conversion of ADP to ATP.
H+
ATP
H+ synthase
Cell wall
H+
H+
H+
ADP ATP
H+
H+
Cell H+ H+ Once inside the cytoplasm, protons combine with O2 to
membrane H+
form water (in aerobic respirers [left]), and with a variety of
With ETS Cytochromes H+
H+
O-containing compounds to produce more reduced compounds.
NAD H O2
SO42–
NO3– Aerobic respiration yields a maximum of 3 ATPs per
oxidized NADH and 2 ATPs per oxidized FADH.
H2 O NO2– HS–
Cytoplasm Anaerobic respiration yields less per NADH and FADH.
Aerobic Anaerobic
respirers respirers](https://image.slidesharecdn.com/ch-7microbialmetabolism-121118161236-phpapp02/85/Ch-7-microbial-metabolism-51-320.jpg)