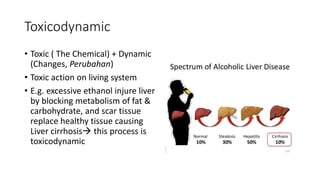
Toxicology is the study of how poisons affect living organisms. Some key points:
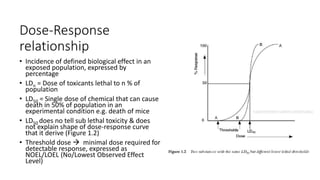
- Toxicology has a long history dating back to the 15th century physician Paracelsus who said "the dose makes the poison."
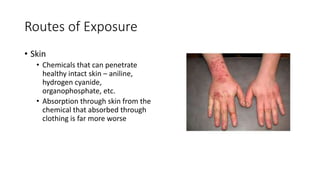
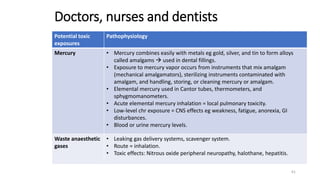
- Toxicants can enter the body through various routes like skin contact, inhalation, or ingestion and cause local or systemic effects.
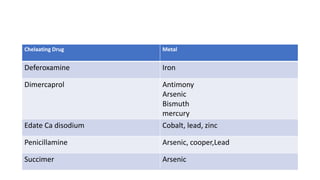
- Toxicants are classified into categories like heavy metals, solvents, radiation, pesticides, and plant/animal toxins. Each can have acute and chronic health effects depending on dose and route of exposure.
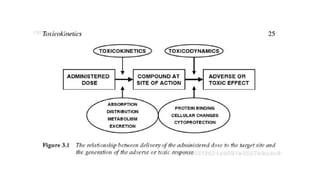
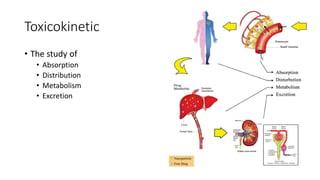
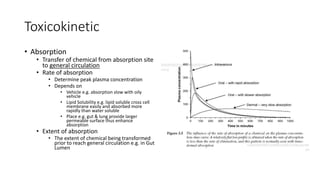
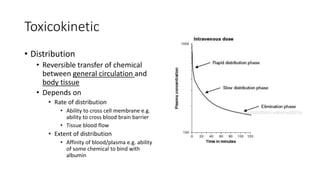
- Toxicokinetics refers to how the body absorbs, distributes, and eliminates toxins while