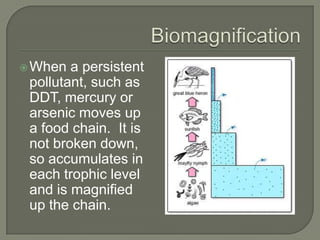
This document provides an introduction to key concepts in pollution for students taking an Environmental Science course. It defines pollution and provides examples of different types of pollution sources. Key terms related to pollution are also defined, such as point source pollution, diffuse pollution, mobile emissions, fugitive emissions, pollution sinks, toxicity, persistence, dispersal, exposure, and bioaccumulation. Examples of different pollutants are requested. Guidelines for participating in an online discussion are also listed.