Embed presentation
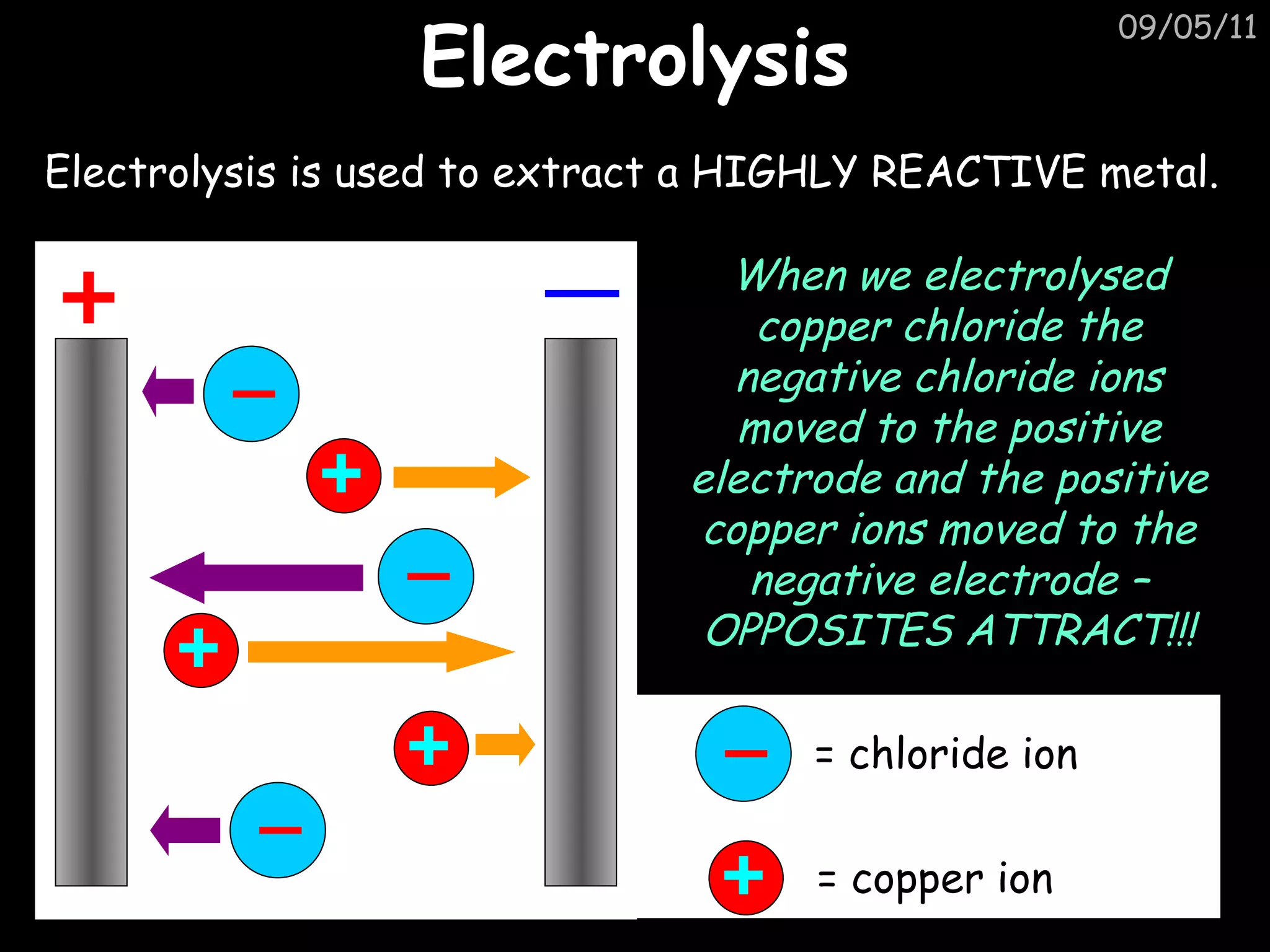
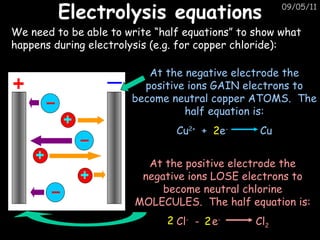

Electrolysis is used to extract reactive metals from their salts. The document explains that during electrolysis of copper chloride, the negative chloride ions move to the positive electrode and the positive copper ions move to the negative electrode. It provides the half reactions that show that at the negative electrode, copper ions gain electrons to form copper atoms, and at the positive electrode, chloride ions lose electrons to form chlorine molecules.

