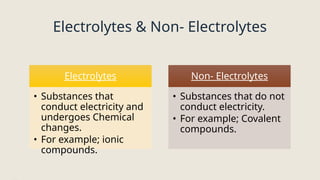
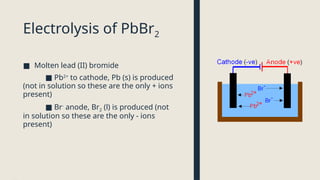
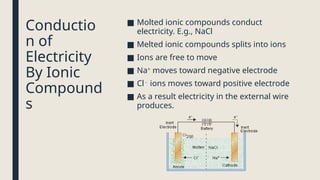
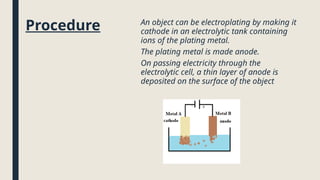
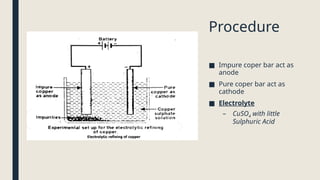
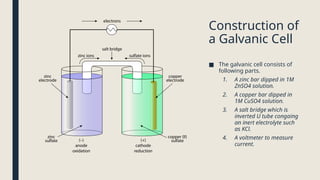
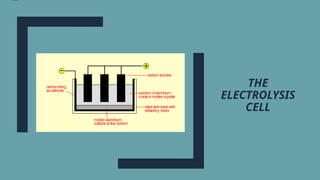
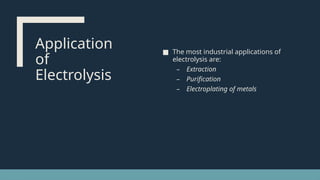
Electrolysis. Definition and procedure of electrolysis, electrolytes and non-electrolytes, electroplating and its procedure, construction and working of Galvanic Cell, Application of electrolysis, O level and FBISE chemistry notes. Chemistry notes for matric.