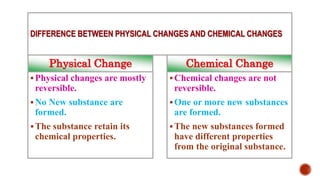
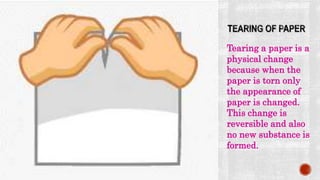
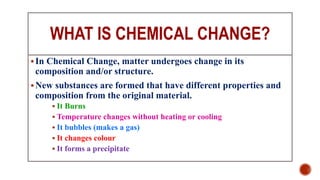
The presentation by Nelson C.S. explains the distinction between physical and chemical changes in matter. Physical changes alter a substance's form without changing its composition, are often reversible, and do not create new substances, whereas chemical changes involve a transformation in composition and produce new substances with different properties. Examples included tearing paper and rusting iron to illustrate physical and chemical changes, respectively.









![CO2 AND [CA(OH)2]
Carbon dioxide reacts with
limewater to form calcium
carbonate, which
precipitates out of the
solution. The carbon
dioxide and limewater
react to produce calcium
carbonate and water. ...
The white milky
suspension/precipitate is
caused by the formation of
calcium carbonate.
Carbon Dioxide (CO2) + Lime
Water [Ca(OH)2] Calcium
Carbonate (CaCO3) + Water
(H2O)](https://image.slidesharecdn.com/physicalandchemicalchanges-201121030602/85/Physical-and-chemical-changes-12-320.jpg)