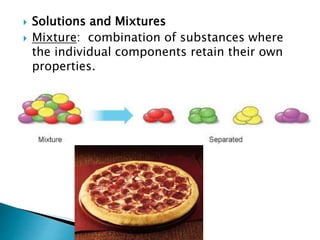
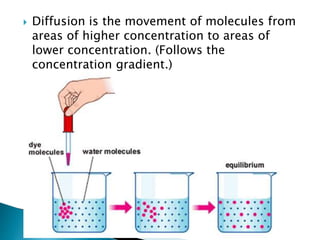
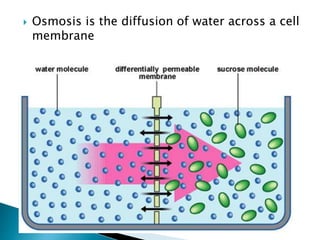
This document provides information on key concepts in earth and space science including:
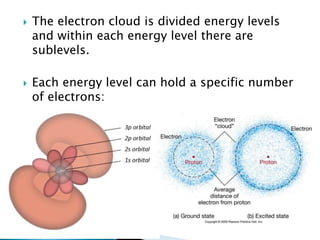
- Atoms are made up of protons, neutrons, and electrons. Protons and neutrons are in the nucleus, while electrons orbit the nucleus.
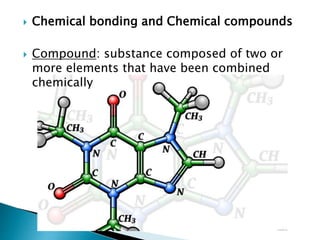
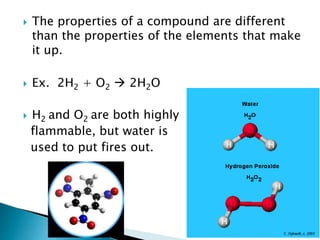
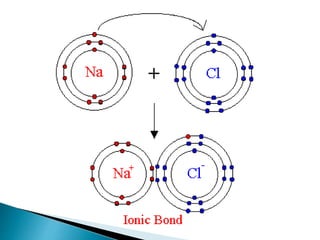
- Isotopes are atoms of the same element that differ in the number of neutrons. Chemical bonds such as covalent and ionic bonds form compounds with different properties than their constituent elements.
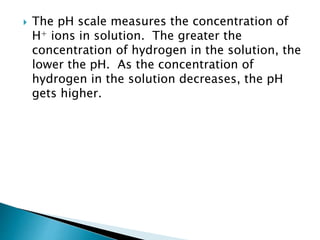
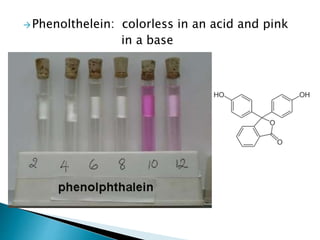
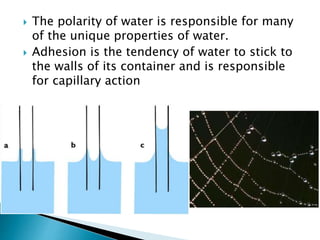
- Acids and bases are defined by their release of hydrogen and hydroxide ions in water, and indicators are used to determine if a solution is acidic or basic. Water has unique properties due to its polarity that allow it to dissolve many substances and transport materials in living things.