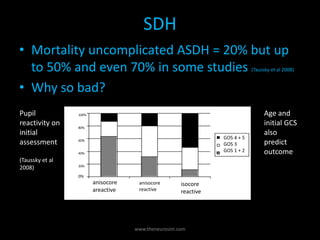
The document provides a comprehensive overview of intracranial hemorrhage, detailing various types such as epidural hematoma (EDH), subdural hematoma (SDH), intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH). It reviews treatment guidelines, outcomes based on different demographics, and the importance of timely surgical intervention while discussing the effects of management strategies on long-term survival. The document emphasizes the need for experience in healthcare settings when treating hemorrhagic strokes to improve patient prognosis.