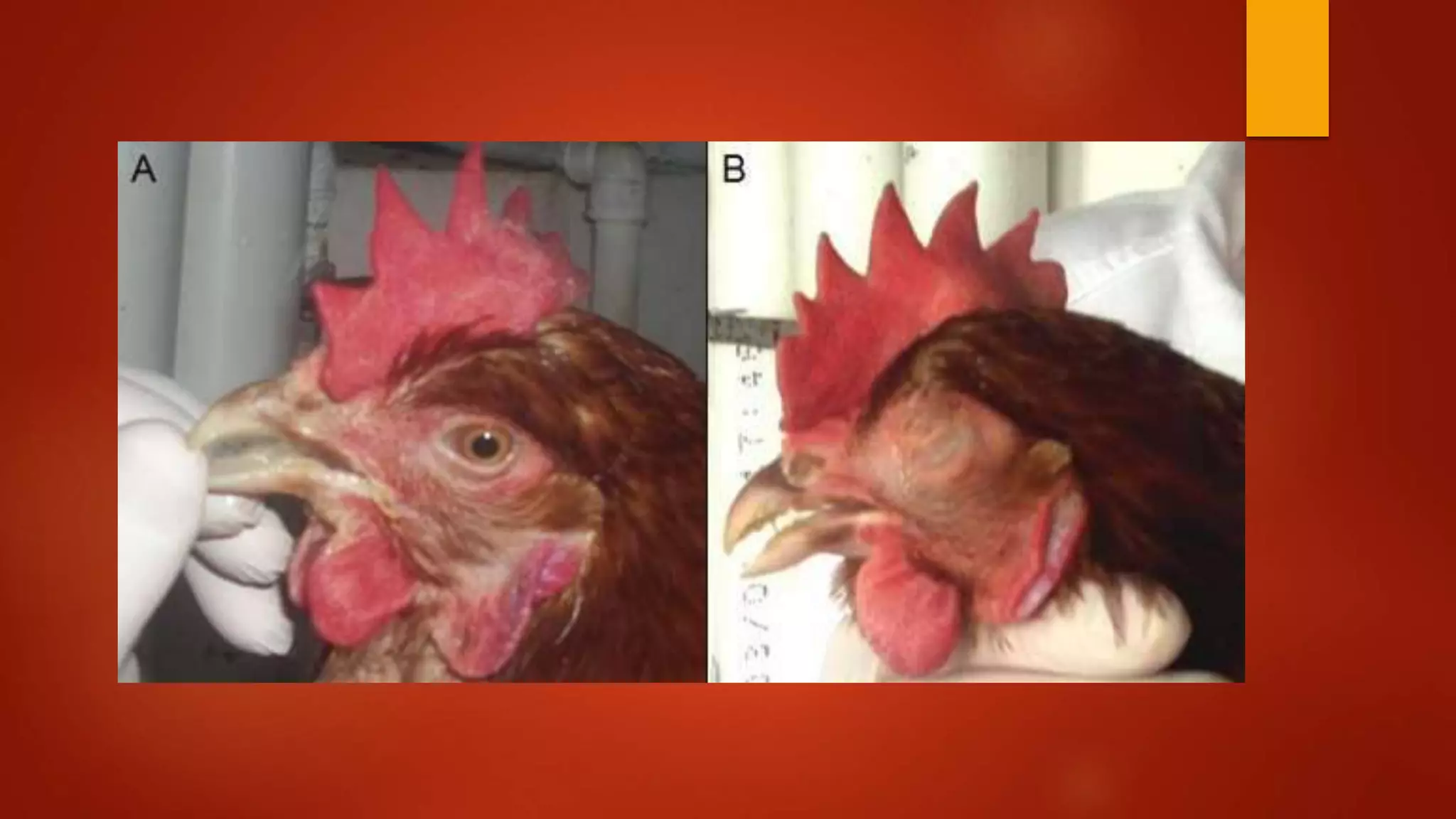
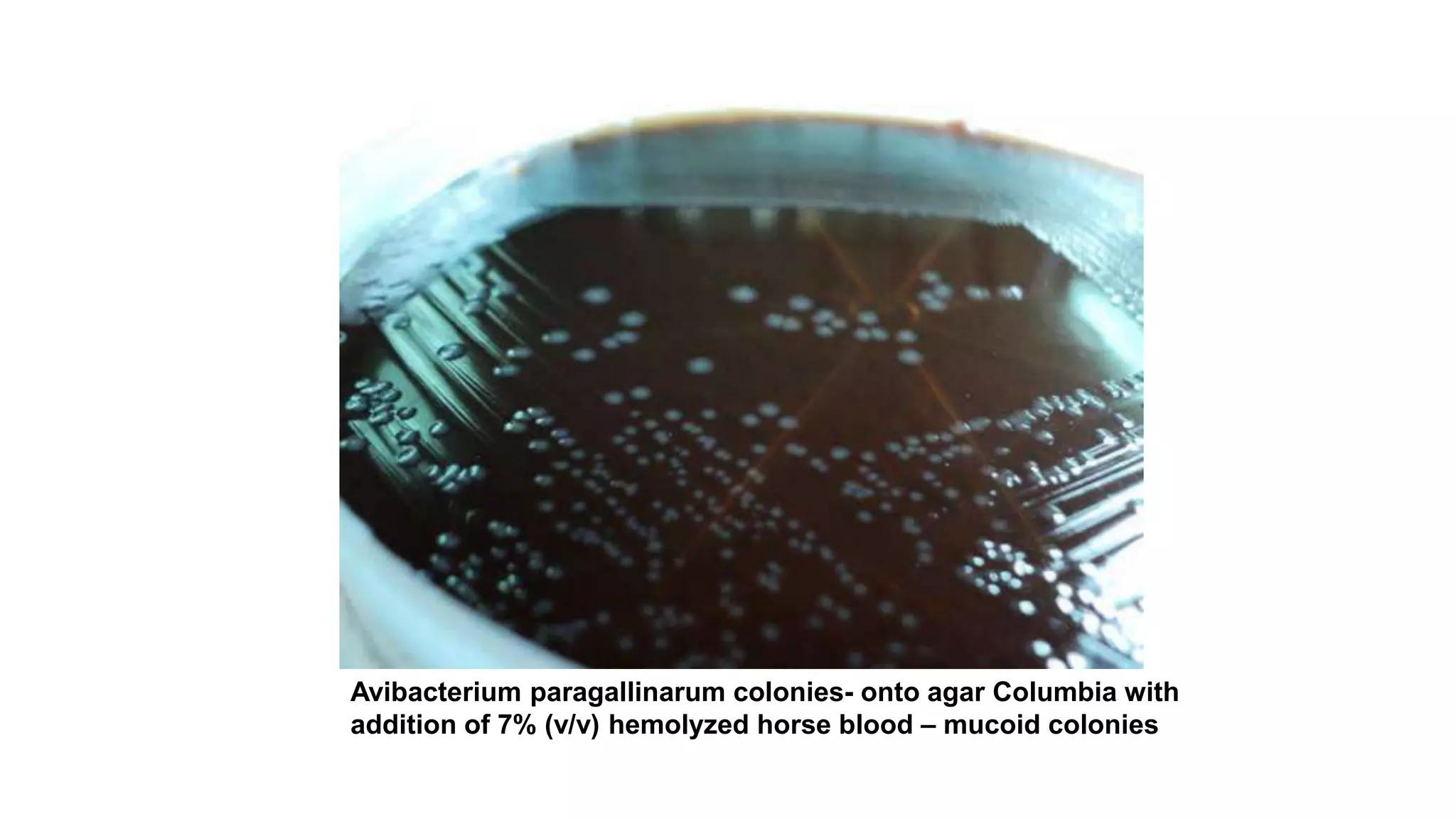
Infectious coryza, caused by Avibacterium paragallinarum, is a highly contagious upper respiratory disease in chickens characterized by nasal discharge, facial swelling, and reduced egg production, leading to significant economic losses. The disease, first diagnosed in 1931, is prevalent globally and primarily transmitted through contaminated environments, affecting all ages of chickens, with older birds showing more severe symptoms. Control measures include sanitation, biosecurity, and vaccination, with antibiotic susceptibility tests indicating resistance to certain antibiotics and sensitivity to others.