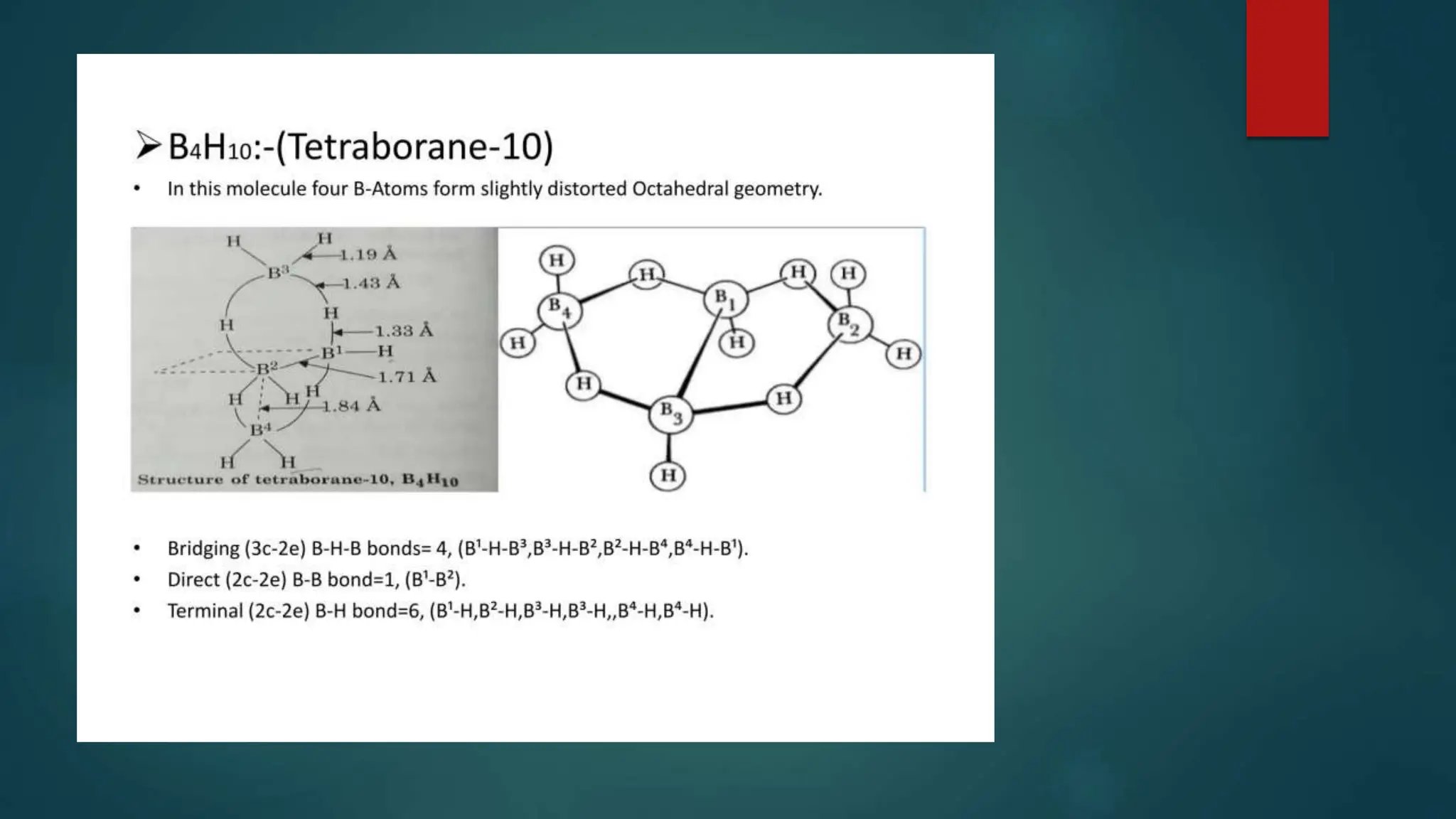
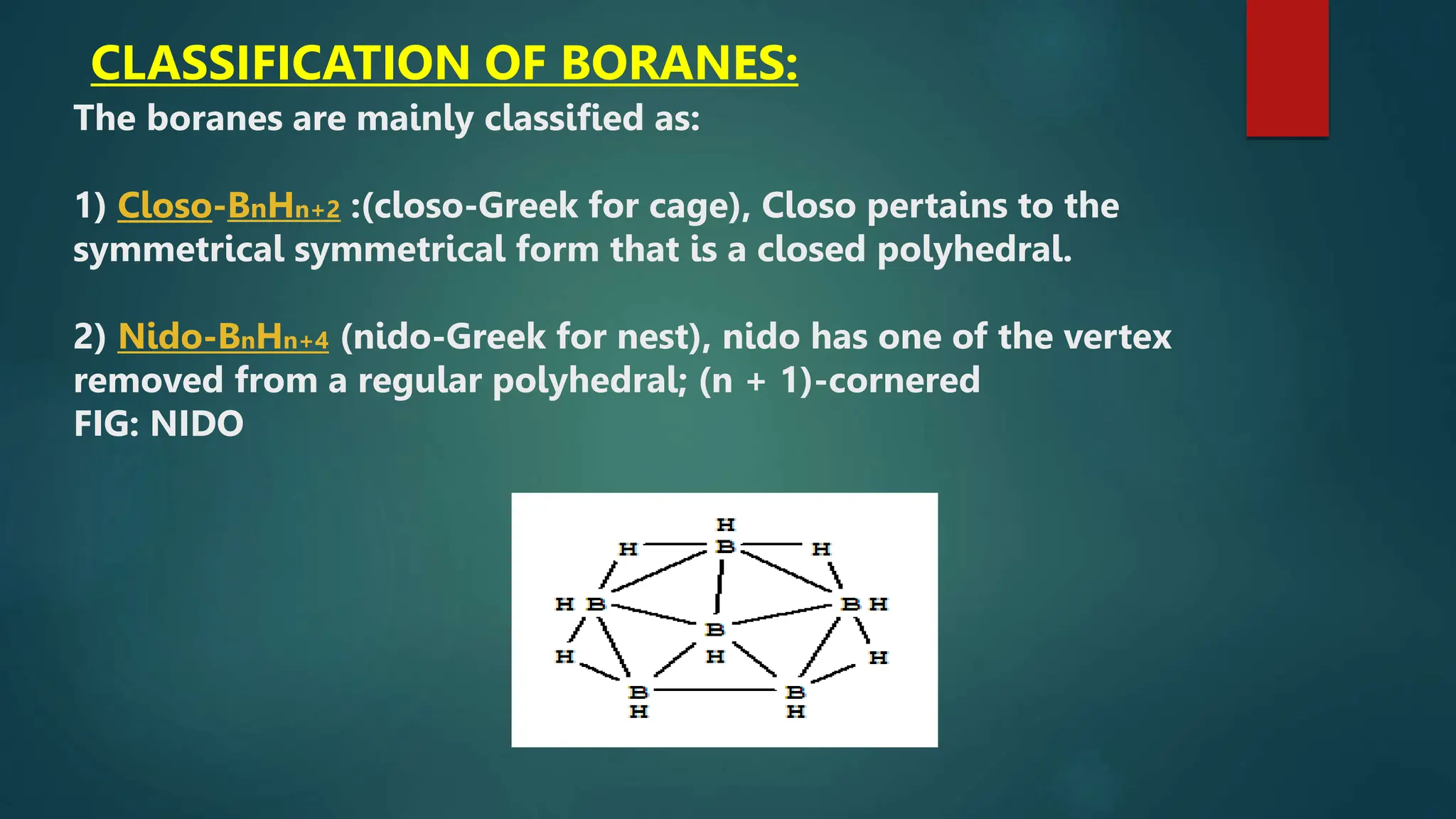
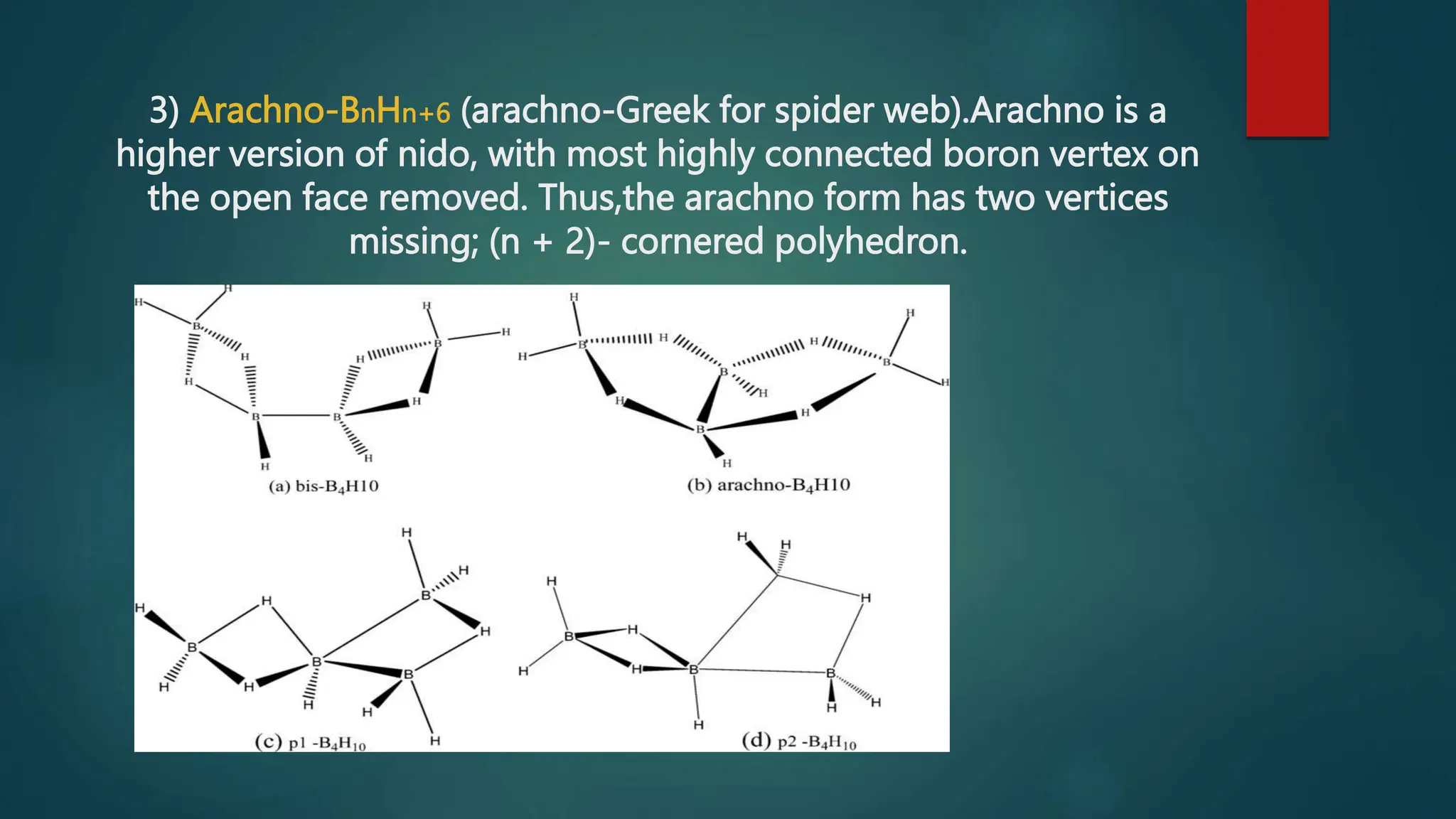
The document discusses higher boranes, their types, structure, bonding, classification, and synthesis. It outlines various types of bonds present in boranes, details the structure of diborane, and categorizes boranes into different groups based on their structures. Additionally, it describes the synthesis methods for diborane, including its reactions and industrial preparation.