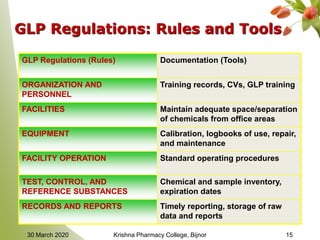
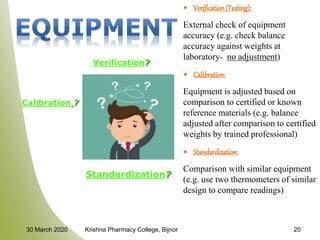
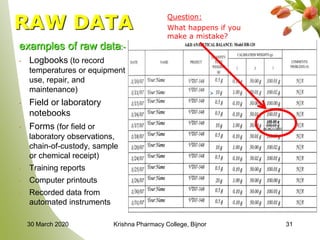
This document discusses Good Laboratory Practice (GLP), a regulatory quality system established by the FDA in the 1970s to ensure the reliability and integrity of non-clinical health and environmental safety studies. It outlines the historical context of GLP, its principles, objectives, and the importance of maintaining high standards in laboratory practices, as well as consequences for non-compliance. The content includes sections on organization, facilities, personnel qualifications, testing protocols, and record-keeping requirements.