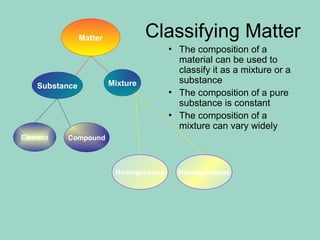
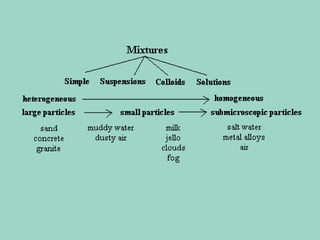
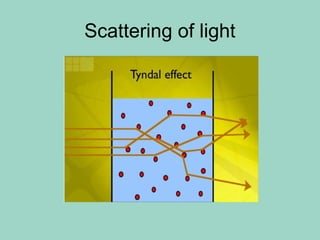
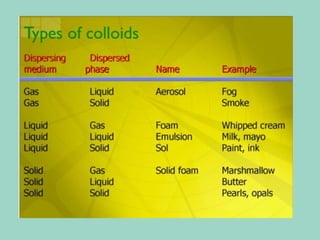
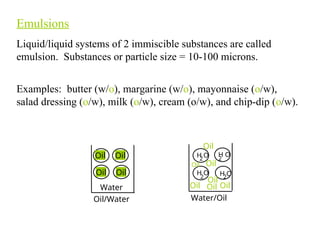
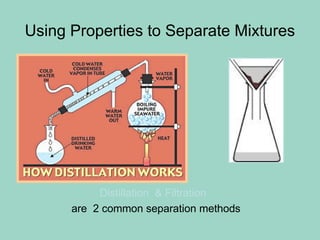
Chapter 6.1 discusses the classification of matter into mixtures and pure substances, emphasizing that pure substances have a constant composition while mixtures can vary. It explains the differences between heterogeneous and homogeneous mixtures, as well as specific types such as suspensions, colloids, and solutions, highlighting their properties and examples. Additionally, it covers separation methods like distillation and filtration for different mixtures.