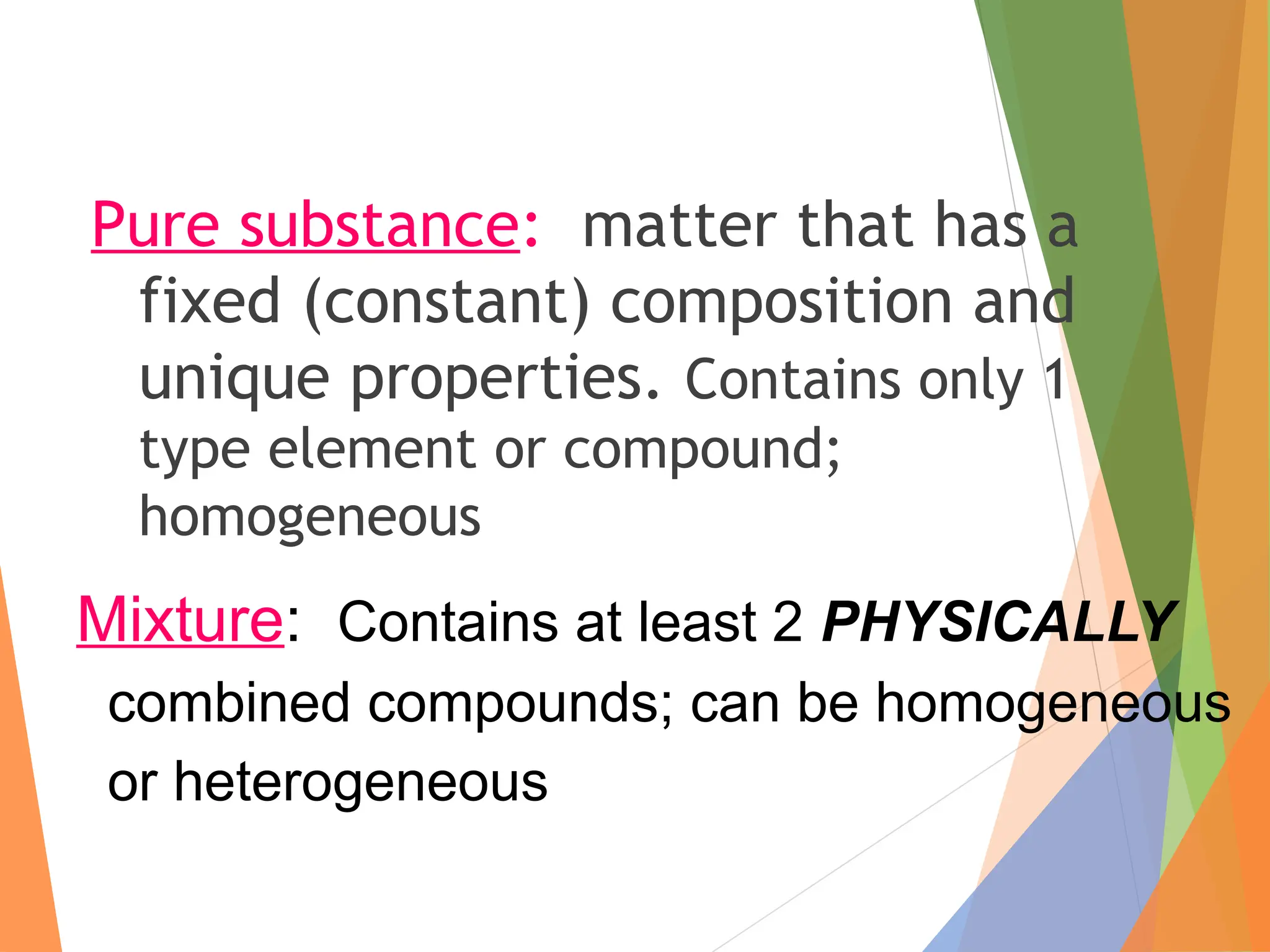
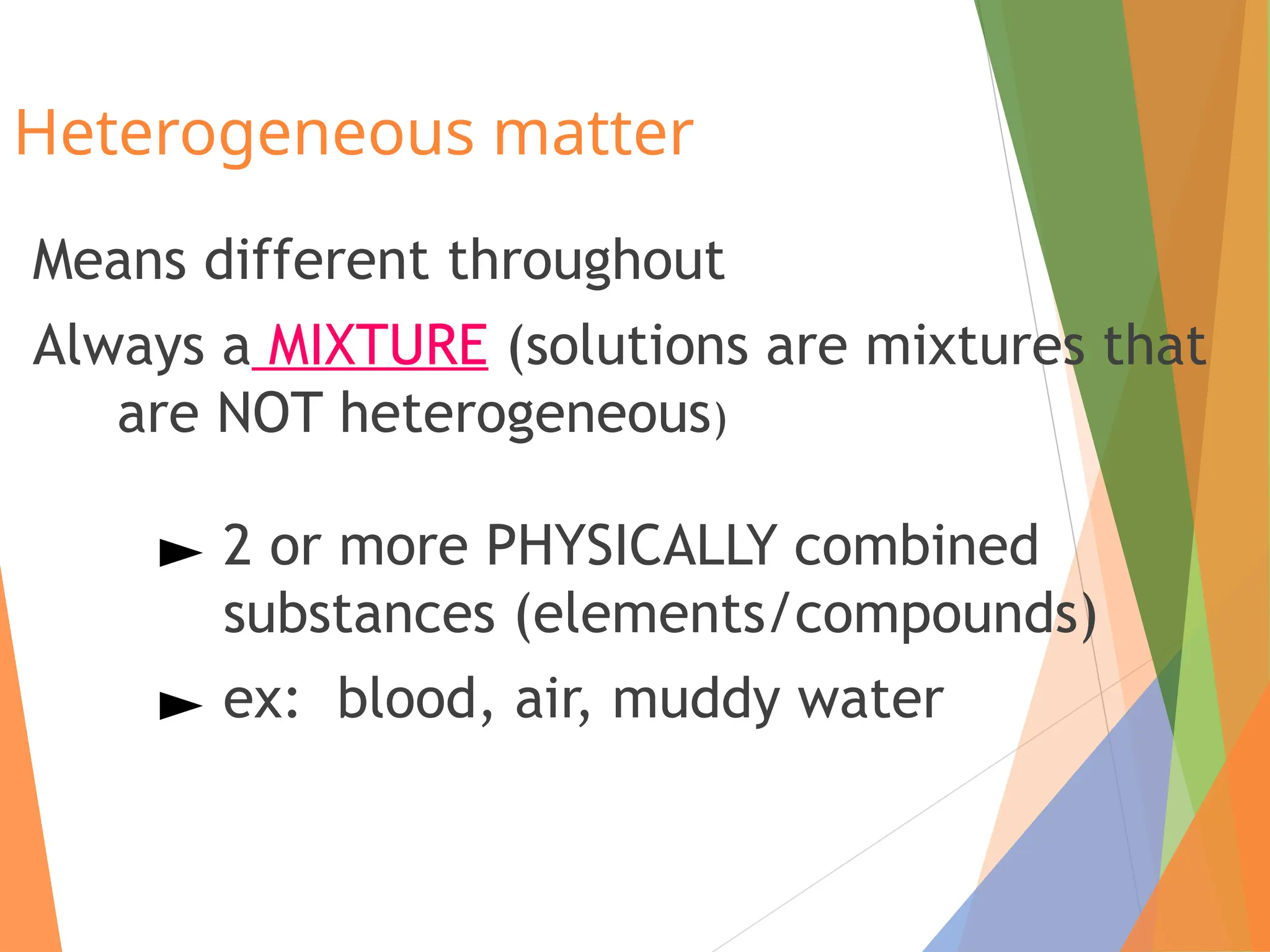
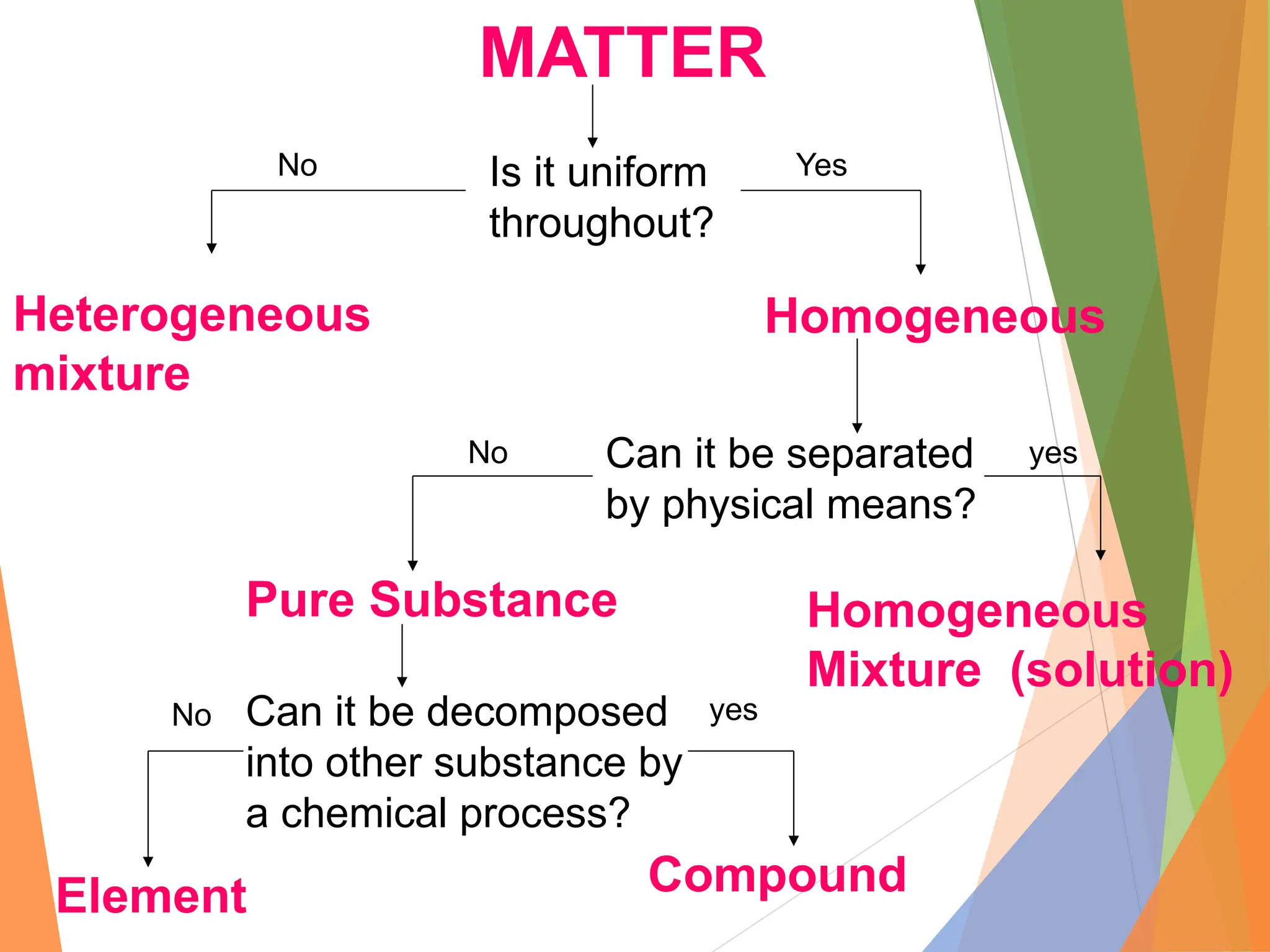
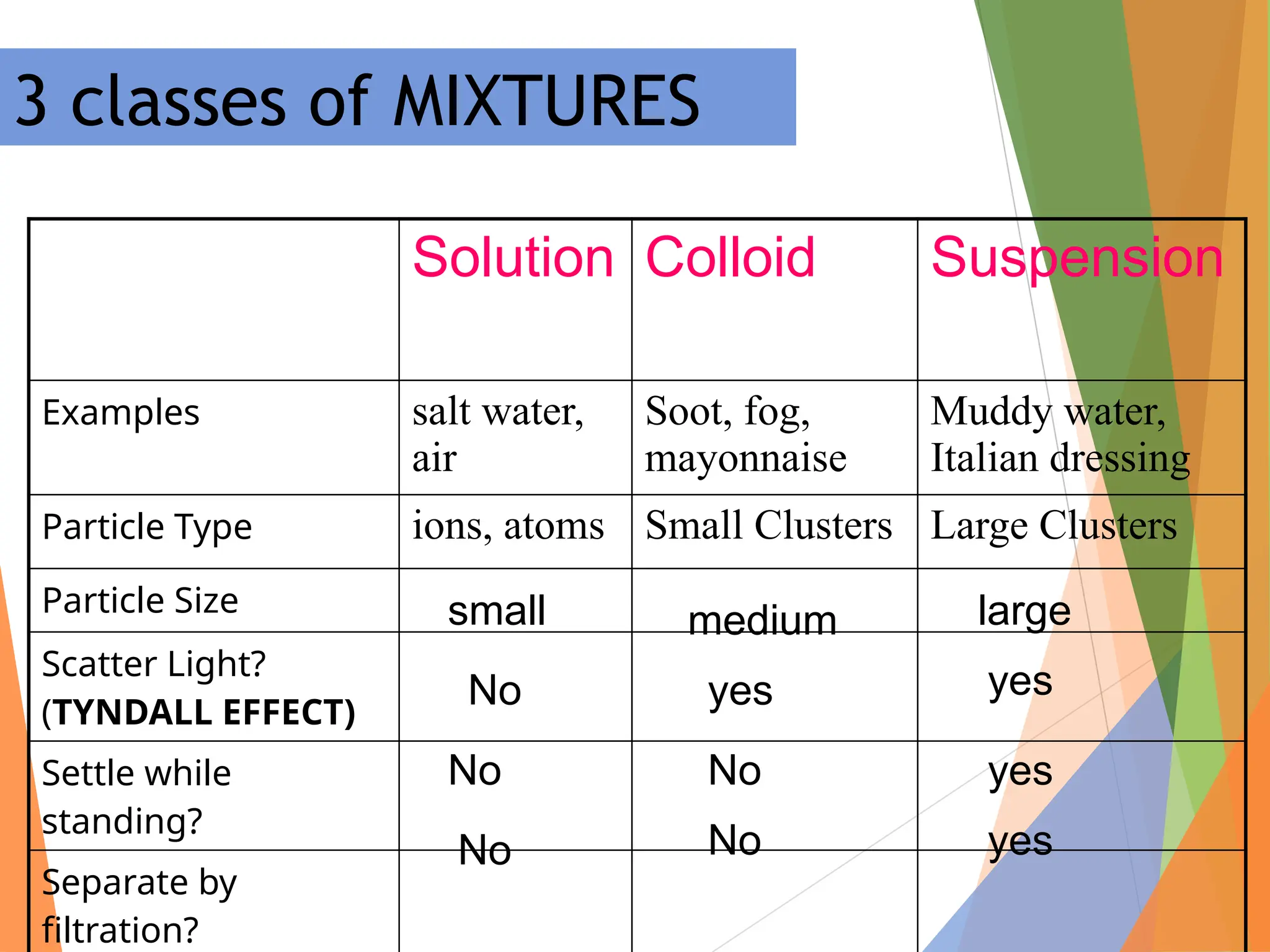
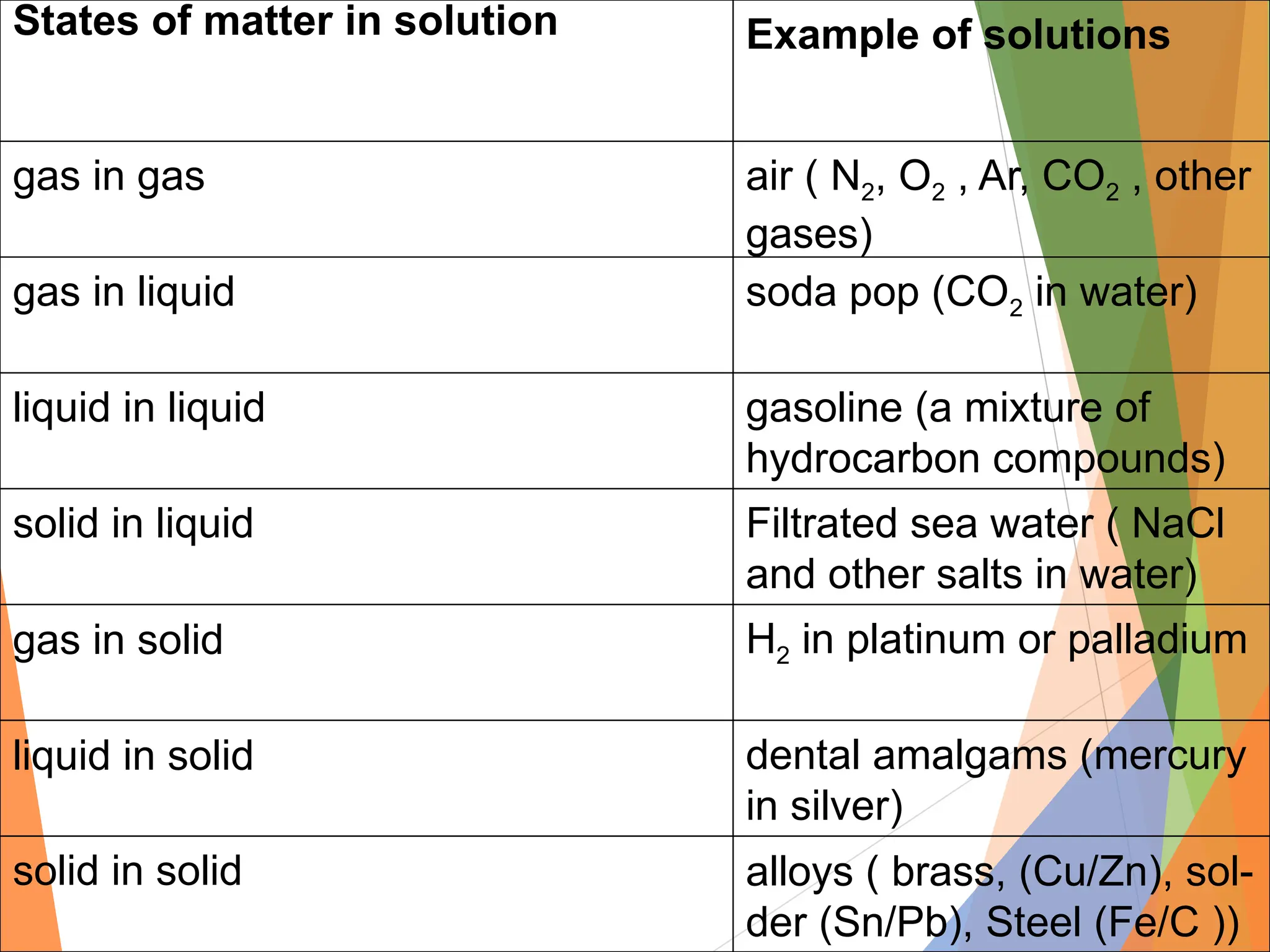
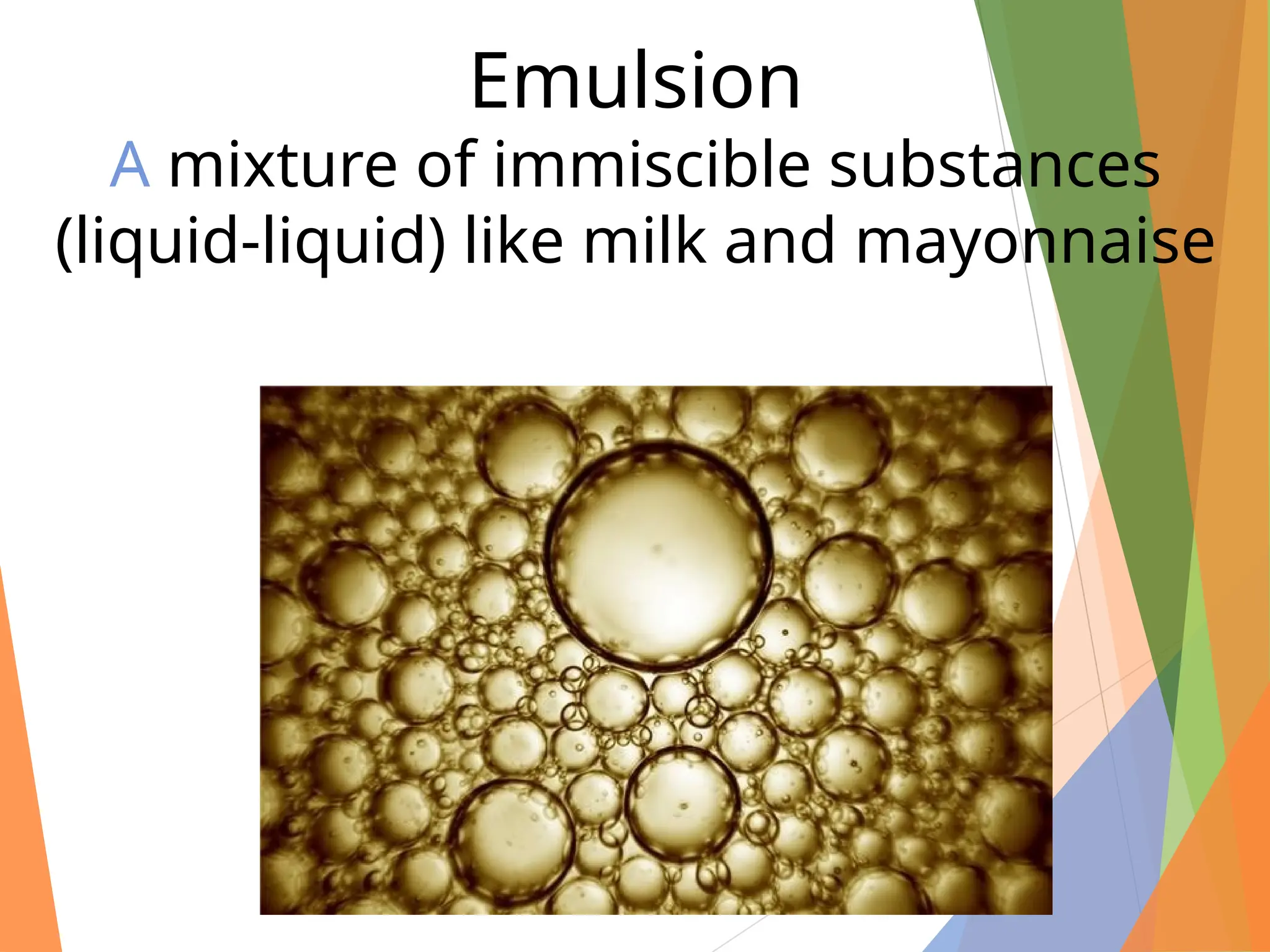
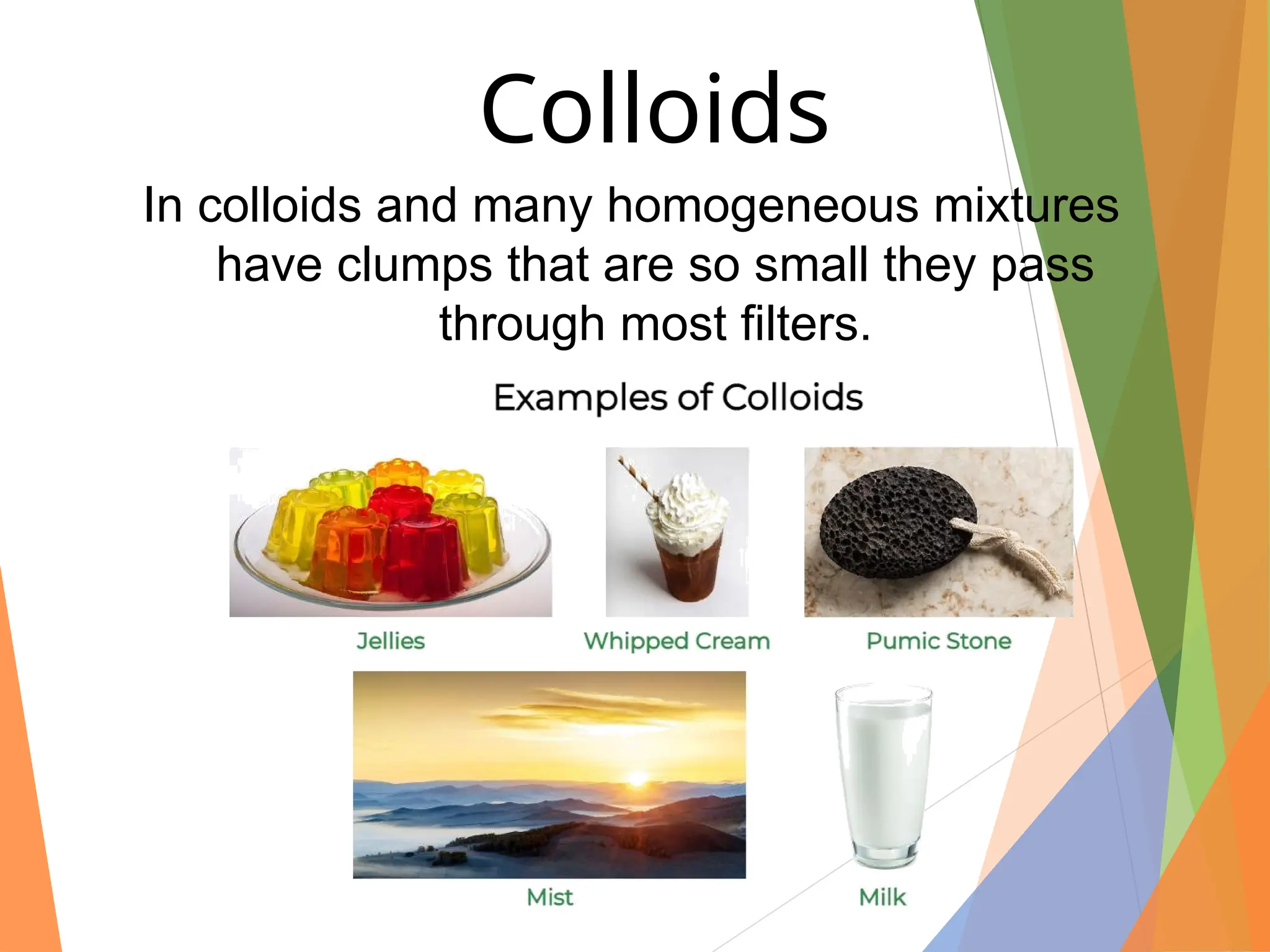
The document distinguishes between pure substances, mixtures, and solutions based on their properties. It defines pure substances as having a fixed composition, while mixtures can be homogeneous or heterogeneous. Additionally, it explains the characteristics of solutions, colloids, and suspensions, including their particle sizes and behaviors, and provides examples for each type.





![[A colloid can be
separated by filtration?]
A.True
B.False](https://image.slidesharecdn.com/g7scienceq1-week4-5-mixturefromsubstancesbasedonproperty-241014045446-dd6711eb/75/G7-Science-Q1-Week-4-5-Mixture-from-Substances-based-on-property-pptx-10-2048.jpg)



![[After passing through a muddy pond, the water
in a stream contains dirt particles. Which of the
following describes the stream?]
A. [solution]
B. [suspension]
C. [pure substance]
D. [colloid]](https://image.slidesharecdn.com/g7scienceq1-week4-5-mixturefromsubstancesbasedonproperty-241014045446-dd6711eb/75/G7-Science-Q1-Week-4-5-Mixture-from-Substances-based-on-property-pptx-15-2048.jpg)




![Which of the following will show the
Tyndall Effect
A.[water]
B.[sugar water]
C.[oxygen gas]
D.[fog]](https://image.slidesharecdn.com/g7scienceq1-week4-5-mixturefromsubstancesbasedonproperty-241014045446-dd6711eb/75/G7-Science-Q1-Week-4-5-Mixture-from-Substances-based-on-property-pptx-21-2048.jpg)

![Which of the following is a colloid
A.[milk]
B. [NaCl in water]
C.[sand and water]
D.[raisin bread]](https://image.slidesharecdn.com/g7scienceq1-week4-5-mixturefromsubstancesbasedonproperty-241014045446-dd6711eb/75/G7-Science-Q1-Week-4-5-Mixture-from-Substances-based-on-property-pptx-24-2048.jpg)